Introduction
Welcome to the DrugBank API! You can use our API to access DrugBank API endpoints, which can get information on drugs, drug products, and drug interactions in our database.
The DrugBank API is organized around REST. Our API has predictable, resource-oriented URLs, and uses HTTP response codes to indicate API errors. We use built-in HTTP features, like HTTP authentication and HTTP verbs, which are understood by off-the-shelf HTTP clients. JSON is returned by all API responses, including errors, although our API libraries convert responses to appropriate language-specific objects.
You can view code examples in the dark area to the right, and you can switch the programming language of the examples with the tabs in the top right.
Selecting your region
The DrugBank API is optionally filtered by region or regions of availability. The URL you use to access the API will determine the drugs, products, etc. that are returned.
If a product / drug appears in the results under a region filter, it means it is available in that region. Once a drug is no longer available (if a drug is withdrawn for example), it will no longer appear in the results.
For example, to search for available drug products by name in Canada, you would use the following URL:
https://api.drugbank.com/v1/drug_names?region=ca&q=abacavir
To search for available drug products by name in Canada or the United States, you would use the following URL:
https://api.drugbank.com/v1/drug_names?region=ca,us&q=abacavir
The currently available regions are:
| Region | Region code |
|---|---|
| Austria | at |
| Canada | ca |
| Colombia | co |
| European Union | eu |
| Indonesia | idn |
| Italy | it |
| Malaysia | my |
| Singapore | sg |
| Thailand | th |
| Turkey | tr |
| United States | us |
| Region | Code | Base URL |
|---|---|---|
| United States | us | https://api.drugbank.com/v1/us |
| Canada | ca | https://api.drugbank.com/v1/ca |
| European Union | eu | https://api.drugbank.com/v1/eu |
Authentication
To authorize, use this code:
# With cURL, you can just pass the correct header with each request
curl -L 'https://api.drugbank.com/v1/endpoint'
-H 'Authorization: myapikey'
Make sure to replace
myapikeywith your development or production API key.
DrugBank uses API keys to allow access to the API. To request an API key please contact us.
All API requests must be made over HTTPS. Calls made over plain HTTP will fail. API requests without authentication will also fail.
DrugBank expects for the API key to be included in all API requests to the server in a header that looks like the following:
Authorization: myapikey
Production Key
Your production key is the authentication token you receive upon sign up. This key does not have a limit in regards to the amount of calls you can make. Any API calls that exceed your quota (if applicable) will be permitted but counted as overage.
Development Key
A development key is a second API key which, unlike the production key, caps the number of allowed requests at 3000 requests/month.
The request limit is to meant to prevent runwaway programs from making calls that exceed your expected usage during the development process. The development key should be kept secret and only be shared with your developers in order to ensure that if a mistake happens during programming, it can be fixed without affecting the production key.
Token Authentication
To get a token that expires in 12 hours, use this code:
curl -L -X POST 'https://api.drugbank.com/v1/tokens' \
-H 'Content-Type: application/json' \
-H 'Authorization: myapikey' \
-H 'Cache-Control: no-cache' -d '{
"ttl": 12
}'
Example command returns JSON structured like this (results may be abbreviated):
{
"token": "eyJhbGciOiJIUzI1NiJ9.eyJrZXlfaWQiOjk5MiwiZXhwIjoxNzE4MDQxNzg1fQ.4K2UNBPlxppfFsO7NHC9hF3qY9LZRq512EYzpiRUDlQ"
}
The following will request a token that expires in 15 minutes:
curl -L -X POST 'https://api.drugbank.com/v1/tokens' \
-H 'Content-Type: application/json' \
-H 'Authorization: myapikey' \
-H 'Cache-Control: no-cache' -d '{
"ttl": "15m"
}'
Example command returns JSON structured like this (results may be abbreviated):
{
"token": "eyJhbGciOiJIUzI1NiJ9.eyJrZXlfaWQiOjk5MiwiZXhwIjoxNzE4MDQxNzg1fQ.4K2UNBPlxppfFsO7NHC9hF3qY9LZRq512EYzpiRUDlQ"
}
To authorize a request using an API token, use this code:
curl -L 'https://api.drugbank.com/v1/endpoint'
-H 'Authorization: Bearer mytoken'
To authorize a browser-based request using an API token, use this code:
const xhr = new XMLHttpRequest();
const url = 'https://api-js.drugbank.com/v1/endpoint';
xhr.open('GET', url);
xhr.setRequestHeader('Authorization', 'Bearer mytoken');
xhr.send();
Alternatively you can use tokens to get access to the DrugBank APIs. Tokens allow short term access to DrugBank API without exposing your secret API key. They are guaranteed to expire within 24 hours. The use of token authentication is required for accessing DrugBank APIs from browser-based applications.
Token life time
The default expiry of a token is 24 hours after it was created. The lifetime of a token can be set with the ttl parameter on creation. When set, the ttl is the number of hours the token is valid for, with a maximum of 24 hours.
If no unit is given, the ttl is assumed to be in hours. The value can also be sent as a string with a unit attached (“m” for minutes or “h” for hours). A ttl of “15m” will request a token that expires in 15 minutes.
Web browser-based application API authentication
Web browser-based applications are permitted to access the DrugBank APIs through:
https://api-js.drugbank.com
All API calls, as described in this documentation, are identical – with the exception of the web address and the authentication method. Token based authentication is required to access the web compatible API.
It is recommend that tokens for use in your web browser-based applications are generated from an internal service that you can authenticate your users against. This way your secret key is not exposed and you can control who has access to your DrugBank subscription.
Pagination
curl -L 'https://api.drugbank.com/v1/drugs?page=2'
-H 'Authorization: myapikey' -v
...
< Link: <https://api.drugbank.com/v1/drugs?page=3&per_page=50>; rel="next",https://api.drugbank.com/v1/drugs?page=1&per_page=50>; rel="prev"
< X-Total-Count: 8221
< X-Per-Page: 50
...
Many API endpoints in the DrugBank API support pagination. Furthermore, to ensure quick response times, it is enabled by default for these endpoints.
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| per_page | 50 | Number of results per page. Any value outside of 1-50 will result in a Bad Request error. |
| page | 1 |
Response Headers
| Header | Description |
|---|---|
| Link | URLs for the next, and prev pages, in the standard Link header format. |
| X-Total-Count | Total number of results available. |
| X-Per-Page | Number of results returned per page. |
Errors
The DrugBank API uses conventional HTTP response codes to indicate the success or failure of an API request. In general, codes in the 2xx range indicate success, codes in the 4xx range indicate an error that failed given the information provided (e.g., a required parameter was omitted), and codes in the 5xx range indicate an error with DrugBank servers (these are rare).
The DrugBank API uses the following error codes:
| Error Code | Meaning |
|---|---|
| 400 | Bad Request – Your request is invalid |
| 401 | Unauthorized – Your API key is wrong |
| 403 | Rate Limit Exceeded – The API is rate limited to 100 requests per second, per client. Try again later. |
| 404 | Not Found – The specified resource could not be found |
| 405 | Method Not Allowed – You tried to access a resource with an invalid method |
| 406 | Not Acceptable – You requested a format that isn’t json |
| 410 | Gone – The resource requested has been removed from our servers |
| 500 | Internal Server Error – We had a problem with our server. Try again later. |
| 503 | Service Unavailable – We’re temporarially offline for maintanance. Please try again later. |
Content Types
Response Body
curl -L 'https://api.drugbank.com/v1/drugs.json'
-H 'Authorization: myapikey'
curl -L 'https://api.drugbank.com/v1/drugs'
-H 'Authorization: myapikey' -H 'Accept: application/json'
In the DrugBank V1 API, response format will default to JSON. At the moment,
this is the only format available. To specify JSON format, you can set the Accept
header to Accept: application/json. You can also use the .json file extension
to specify that json encoding is desired.
Request Headers
| Header | Description |
|---|---|
| Accept | Requested MIME type of response. |
Response Headers
| Header | Description |
|---|---|
| Content-Type | MIME type of the response body. |
Request Body
curl -L -X POST "https://api.drugbank.com/v1/ddi" \
-H "Content-Type: application/json" \
-H 'Authorization: myapikey' \
-H "Cache-Control: no-cache" -d '{
"ndc": ["0143-9503", "0056-0173"],
"drugbank_id": ["DB00503", "DB01221", "DB00331", "DB00819"],
"product_concept_id": ["DBPC0024484", "DBPC0085378"]
}'
For API calls which use a POST request to send data to the DrugBank API
(such as when finding drug-drug interactions with mixed input),
the request must set an appropriate Content-Type header.
Request Headers
| Header | Description |
|---|---|
| Content-Type | MIME type of request body. |
Drug Search / Autocomplete
Medication Search Plugin
Our medication search plugin provides a “plug and play” alternative to implementing our medication name search endpoint:
As a standard web component, it is easy to integrate and style to suit your needs and comes with a number of available properties to fine-tune its functionality. This plugin can be used together with our Drug-Drug Interactions Plugin. For more information, please see this public repository.
Medication Name Search
curl -L 'https://api.drugbank.com/v1/product_concepts?q=acetamino&hit_details=true'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"hits": [
{
"value": "<em>Acetamino</em>phen"
},
{
"value": "<em>ACETAMINO</em>FEN JARABE"
}
],
"detailed_hits": [
{
"field": "title",
"matches": [
"<em>Acetamino</em>phen"
],
"match_highlight_name": "<em>Acetamino</em>phen"
},
{
"field": "ingredients",
"matches": {
"DB00316": [
"<em>Acetamino</em>phen"
]
},
"match_highlight_name": "<em>Acetamino</em>phen"
},
"..."
],
"name": "Acetaminophen",
"display_name": null,
"drugbank_pcid": "DBPC0180857",
"brand": null,
"level": 1,
"route": null,
"form": null,
"strengths": null,
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1950-01-01",
"regions": {
"us": true,
"canada": true,
"eu": false,
"germany": true,
"colombia": true,
"turkey": true,
"italy": true,
"malaysia": true,
"austria": true,
"indonesia": true,
"thailand": true,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [
{
"name": "acetaminophen",
"RXCUI": "161"
}
],
"ingredients": [
{
"name": "Acetaminophen",
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
}
}
]
}
]
Medication name search allows you to quickly search for product concepts.
Like the most of the DrugBank API, the returned concepts can be filtered by region using a the region parameter like /v1/product_concepts?region=us.
By default, the search returns only top-level product concepts - concepts without route, form or strength information. Lower level concepts can be found using the level, min_level, and max_level parameters.
Search is performed by passing in a full-text search parameter q. The search results are suitable for search-as-you-type. The query parameter is used to search brand names, ingredient names and synonyms as well as ingredient names and synonyms. When query_type=advanced is used, basic boolean operations are possible in the string. Alternatively, query_type=exact can be used to return only concepts that contain exact matches to the query string.
Each result includes a hits array, which lists the best matched descriptors (brand, ingredient, and/or product names and synonyms) for that concept. These are useful for facilitating search-as-you-type queries. Adding the hit_details=true parameter will also return a detailed_hits array that gives further information on how the matches were obtained.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts?q=<search_term>
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | The text-search query string. | |
| query_type | simple | If set to advanced, allows basic boolean operations in the query parameter q. If set to exact, only returns product concepts containing an exact match to the query string q. |
| hit_details | false | If set to true, returns additional information on matched brands/ingredients/products for the concept. |
| level | The product concept level to filter by (optional). | |
| min_level | The minimum product concept level to return (optional). | |
| max_level | The maximum product concept level to return (optional). | |
| unbranded_only | false | If true, returns only product concepts without an associated brand. |
| always_ingredients | true | If true, only returns product concepts with ingredients. Set to false to return all product concepts. |
| include_allergens | false | Include allergen product concepts in the results. |
| include_vaccines | true | Include vaccine product concepts in the results. |
| withdrawn | false | If set to include, includes product concepts for which all products have been withdrawn. If set to true, includes only product concepts for which all products have been withdrawn. If set to false, includes only product concepts with at least one non-withdrawn product. |
| include_simple_desc | false | If set to true, include simple descriptions for the product concept ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product concept ingredients. |
Example Queries
| Query | Type | Description |
|---|---|---|
| lido | simple | Product concepts which contain “lido” in the search terms (eg: “Lidocaine”). |
| abac -sulf | advanced | Product concepts which contain “abac” but not “sulf” (eg: “Abacavir” but not “Abacavir Sulfate”). |
| abac -sulf | simple | Product concepts which contain “abac” and “-sulf” (“Abacavir-sulfate”). |
| abac lam | simple | Product concepts which contain both “abac” and “lam” (eg: “Abacavir / Lamivudine”). |
Drug Name Search
Drug name search returns a list of drugs/product information suitable for use with autocomplete forms, for quickly finding the right drugs/products.
curl -L 'https://api.drugbank.com/v1/drug_names?q=lipitor'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"products": [
{
"hits": [
{
"field": "name",
"value": "<em>Lipitor</em>"
},
{
"field": "prescribable_name",
"value": "<em>Lipitor</em> 80 mg Oral Tablet"
}
],
"name": "Lipitor",
"prescribable_name": "Lipitor 80 mg Oral Tablet",
"country": "Canada",
"ndc_product_codes": null,
"dpd_codes": [
"02243097"
],
"ema_product_codes": null,
"ema_ma_numbers": null,
"pzns": [],
"co_product_ids": [],
"tr_product_ids": [],
"it_product_ids": [],
"my_product_ids": [],
"at_product_ids": [],
"idn_product_ids": [],
"th_product_ids": [],
"sg_product_ids": [],
"mx_registration_ids": [],
"mx_product_ids": [],
"dosage_form": "Tablet",
"strength": {
"number": "80",
"unit": "mg"
},
"route": "Oral",
"approved": true,
"unapproved": false,
"generic": false,
"otc": false,
"mixture": false,
"allergen": false,
"vaccine": false,
"ingredients": [
{
"drugbank_id": "DB01076",
"name": "Atorvastatin",
"cas": "134523-00-5",
"strength": {
"number": "80",
"unit": "mg"
}
}
],
"images": []
},
{
"hits": [
{
"field": "name",
"value": "<em>Lipitor</em>"
},
{
"field": "prescribable_name",
"value": "<em>Lipitor</em> 20 mg Oral Tablet"
}
],
"name": "Lipitor",
"prescribable_name": "Lipitor 20 mg Oral Tablet",
"rx_norm_prescribable_name": "Lipitor 20 MG Oral Tablet",
"country": "US",
"ndc_product_codes": [
"00071-0156",
"49299-1091",
"..."
],
"dpd_codes": null,
"ema_product_codes": null,
"ema_ma_numbers": null,
"pzns": [],
"co_product_ids": [],
"tr_product_ids": [],
"it_product_ids": [],
"my_product_ids": [],
"at_product_ids": [],
"idn_product_ids": [],
"th_product_ids": [],
"sg_product_ids": [],
"mx_registration_ids": [],
"mx_product_ids": [],
"dosage_form": "Tablet, film coated",
"strength": {
"number": "20",
"unit": "mg/1"
},
"route": "Oral",
"approved": true,
"unapproved": false,
"generic": false,
"otc": false,
"mixture": false,
"allergen": false,
"vaccine": false,
"ingredients": [
{
"drugbank_id": "DB01076",
"name": "Atorvastatin",
"cas": "134523-00-5",
"strength": {
"number": "20",
"unit": "mg/1"
}
}
],
"images": [
{
"ndc_id": "49299-1091",
"description": "atorvastatin 20 MG Oral Tablet [Lipitor]",
"image_url_original": "//s3-us-west-2.amazonaws.com/drugbank/product_images/images/original/49299-109120180906-25352-ie7nxm.jpg?1536257345",
"image_url_tiny": "//s3-us-west-2.amazonaws.com/drugbank/product_images/images/tiny/49299-109120180906-25352-ie7nxm.jpg?1536257345",
"image_url_thumb": "//s3-us-west-2.amazonaws.com/drugbank/product_images/images/thumb/49299-109120180906-25352-ie7nxm.jpg?1536257345",
"image_url_medium": "//s3-us-west-2.amazonaws.com/drugbank/product_images/images/medium/49299-109120180906-25352-ie7nxm.jpg?1536257345"
},
{
"ndc_id": "54569-4467",
"description": "atorvastatin 20 MG Oral Tablet [Lipitor]",
"image_url_original": "//s3-us-west-2.amazonaws.com/drugbank/product_images/images/original/54569-446720180819-11162-7871u7.jpg?1534669642",
"image_url_tiny": "//s3-us-west-2.amazonaws.com/drugbank/product_images/images/tiny/54569-446720180819-11162-7871u7.jpg?1534669642",
"image_url_thumb": "//s3-us-west-2.amazonaws.com/drugbank/product_images/images/thumb/54569-446720180819-11162-7871u7.jpg?1534669642",
"image_url_medium": "//s3-us-west-2.amazonaws.com/drugbank/product_images/images/medium/54569-446720180819-11162-7871u7.jpg?1534669642"
},
"..."
]
},
"..."
]
}
HTTP Request
GET https://api.drugbank.com/v1/drug_names
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | null | The string you want to search with. |
| fuzzy | false | If set to true, enable fuzzy search (see fuzzy searching below). |
| include_allergens | false | If set to true, include allergen products in the results. |
| include_vaccines | true | If set to true, include vaccine products in the results. |
| include_simple_desc | false | If set to true, include simple descriptions for the product ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product ingredients. |
Notice the hits array returned in the results. The hits contain highlighted
snippets from the match. You can use these highlights in autocomplete applications.
The matching part of the text is wrapped in an <em> tag, which you can style
as you wish in your application. Note that the hits only contain the product name, and thus will
not have a highlighted snippet if the query matches an ingredient not listed in the product name.
Prescribable Name Search
curl -L 'https://api.drugbank.com/v1/drug_names/simple?q=lipitor'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"products": [
{
"hits": [
{
"field": "name",
"value": "<em>Lipitor</em> 10 mg Oral Tablet"
}
],
"name": "Lipitor 10 mg Oral Tablet",
"rx_norm_name": "Lipitor 10 MG Oral Tablet",
"country": "US",
"brands": [
"Lipitor"
],
"ndc_product_codes": [
"00071-0155",
"49299-1090",
"..."
],
"dpd_codes": null,
"ema_product_codes": null,
"ema_ma_numbers": null,
"pzns": [],
"co_product_ids": [],
"tr_product_ids": [],
"it_product_ids": [],
"my_product_ids": [],
"at_product_ids": [],
"idn_product_ids": [],
"th_product_ids": [],
"sg_product_ids": [],
"mx_registration_ids": [],
"mx_product_ids": [],
"dosage_forms": [
"Tablet, film coated"
],
"strength_number": "10",
"strength_unit": "mg/1",
"dosage_form": [
"Tablet, film coated"
],
"route": "Oral",
"approved": true,
"unapproved": false,
"generic": false,
"otc": false,
"mixture": false,
"allergen": false,
"vaccine": false,
"ingredients": [
{
"drugbank_id": "DB01076",
"name": "Atorvastatin",
"cas": "134523-00-5",
"strength_number": "10",
"strength_unit": "mg/1"
}
]
},
{
"hits": [
{
"field": "name",
"value": "<em>Lipitor</em> 20 mg Oral Tablet"
}
],
"name": "Lipitor 20 mg Oral Tablet",
"country": "Malaysia",
"brands": [
"LIPITOR TABLET 20MG"
],
"ndc_product_codes": null,
"dpd_codes": null,
"ema_product_codes": null,
"ema_ma_numbers": null,
"pzns": [],
"co_product_ids": [],
"tr_product_ids": [],
"it_product_ids": [],
"my_product_ids": [
"MAL20034211ARZ"
],
"at_product_ids": [],
"idn_product_ids": [],
"th_product_ids": [],
"sg_product_ids": [],
"mx_registration_ids": [],
"mx_product_ids": [],
"dosage_forms": [
"Tablet"
],
"strength_number": "20",
"strength_unit": "MG",
"dosage_form": [
"Tablet"
],
"route": "Oral",
"approved": true,
"unapproved": false,
"generic": false,
"otc": false,
"mixture": false,
"allergen": false,
"vaccine": false,
"ingredients": [
{
"drugbank_id": "DB01076",
"name": "Atorvastatin",
"cas": "134523-00-5",
"strength_number": "20",
"strength_unit": "MG"
}
]
},
"..."
]
}
Prescribable name (simple) search uses the concept of a prescribable name, the normalized, most common name used to describe a medication. The results of this search will not contain multiple brand names for a given drug. Instead the prescribable name will be unique for a dosage strength/form and a name. Product codes and brand names will be listed for each matched prescribable name.
This type of search is useful when logging medications a customer/patient may
be taking. You can combine a name and a dosage strength to quickly filter your
search. For example searching for lipitor 20 mg will quickly return the most
likely drug that the individual is taking.
HTTP Request
GET https://api.drugbank.com/v1/drug_names/simple
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | null | The string you want to search with. |
| fuzzy | false | If set to true, enable fuzzy search (see fuzzy searching below). |
| include_allergens | false | If set to true, include allergen products in the results. |
| include_vaccines | true | If set to true, include vaccine products in the results. |
| include_simple_desc | false | If set to true, include simple descriptions for the product ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product ingredients. |
Notice the hits array returned in the results. The hits contain highlighted
snippets from the match. You can use these highlights in autocomplete applications.
The matching part of the text is wrapped in an <em> tag, which you can style
as you wish in your application. Note that the hits only contain the prescribable name, and thus will not have a highlighted snippet if the query matches an ingredient not listed in the prescribable name.
Ingredient Name Search
curl -L 'https://api.drugbank.com/v1/ingredient_names?q=acetaminophen'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"hits": [
{
"field": "name.autocomplete",
"value": "<em>Acetaminophen</em>"
},
{
"field": "synonyms",
"value": "<em>Acetaminophen</em>"
}
],
"drugbank_id": "DB00316",
"name": "Acetaminophen",
"cas_number": "103-90-2"
}
]
This endpoint searches and returns drug ingredients only. The results of this search will not contain drug product information, such as brand names or dosage strength/form (see Drug Name Search or Prescribable Name Search). This type of search is useful for looking up information for compounding drugs, drug products outside of our covered regions, or to quickly find the DrugBank ID and simple descriptions for a drug ingredient.
HTTP Request
GET https://api.drugbank.com/v1/ingredient_names
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | null | The string you want to search with. |
| fuzzy | false | If set to true, enable fuzzy search (see fuzzy searching below). |
| include_simple_desc | false | If set to true, include simple descriptions for the ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the ingredients. |
Notice the hits array returned in the results. The hits contain highlighted
snippets from the match. You can use these highlights in autocomplete applications.
The matching part of the text is wrapped in an <em> tag, which you can style
as you wish in your application. Note that the hits encompass the drug ingredient in
question, any salts thereof, and any synonyms captured within DrugBank.
Fuzzy Searching
This example demonstrates a misspelling of “Advil”, with fuzzy search enabled you will still get a result (try it yourself!).
curl -L 'https://api.drugbank.com/v1/drug_names?q=addvil&fuzzy=true'
-H 'Authorization: myapikey'
Fuzzy searching allows for misspellings, but is not enabled by default,
you must set fuzzy=true. By setting fuzzy=true you are telling the API to
allow a certain number of misspellings to still count as a match (defaults to 2).
You can also pass a number of misspellings (0, 1, or 2) in to tailor the
likelihood of a match (for example, fuzzy=1 will allow 1 misspelled letter).
Products
Common Parameters
The following parameters can be applied when retrieving any product.
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| drug_details | false | If true, returns the full details for the drug ingredients, otherwise just the drug name and DrugBank ID are returned. Requires access to the Drugs endpoint to use. Note that if used, the include_simple_desc and include_clinical_desc parameters will be ignored since the descriptions are already included in the drug details. |
| include_simple_desc | false | If set to true, include simple descriptions for the product ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product ingredients. |
| include_references | false | If true, includes the lists of references for the drug ingredients. Note that you also have to specify drug_details=true to see this data. See References for details. |
Related Products
Related products can be retrieved by appending an endpoint to the url in the following format:
https://api.drugbank.com/v1/products/<ID>/related/<type>
For example:
https://api.drugbank.com/v1/products/<ID>/related/generics
| Type | Description |
|---|---|
| generics | Returns a list of generic i.e. unbranded versions of the product. |
Regional Products
Products are identified in different ways in different regions. Here is the list of unique identifier fields for all the currently supported regions.
| Region | Region Code | Product ID Field | Local Product ID | Example |
|---|---|---|---|---|
| Austria | at | at_product_id | MA number | 16965 |
| Canada | ca | dpd | DIN | 2341093 |
| Colombia | co | co_product_id | Invima ID | INVIMA M-012422 |
| European Union | eu | ema_ma_number | EU MA number | EU/1/04/276/001 |
| European Union | eu | ema_product_code | Product number | EMEA/H/C/000471 |
| Indonesia | idn | idn_product_id | Nomor registrasi | GKL0122234837A1 |
| Italy | it | it_product_id | AIC | 038835056 |
| Malaysia | my | my_product_id | Registration no. | MAL12115054ACZ |
| Singapore | sg | sg_product_id | Licence no. | SIN11734P |
| Thailand | th | th_product_id | Registration no. | 1C 95/60(N) |
| Turkey | tr | tr_product_id | Barcode | 8699508150243 |
| United States | us | ndc | 9-digit NDC | 0169-5174 |
Get a specific regional product
Get a specific US product
curl -L 'https://api.drugbank.com/v1/products/50090-5181?drug_details=true'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"ndc_product_code": "50090-5181",
"originator_ndc_product_code": "50090-5181",
"dpd_id": null,
"ema_product_code": null,
"ema_ma_number": null,
"pzn": null,
"co_product_id": null,
"tr_product_id": null,
"it_product_id": null,
"my_product_id": null,
"at_product_id": null,
"idn_product_id": null,
"th_product_id": null,
"sg_product_id": null,
"mx_registration_id": null,
"mx_product_id": null,
"name": "Atorvastatin Calcium",
"prescribable_name": "Atorvastatin calcium trihydrate 20 mg Oral Tablet",
"rx_norm_prescribable_name": "atorvastatin calcium 20 MG Oral Tablet",
"started_marketing_on": "2018-12-23",
"ended_marketing_on": null,
"approved_on": null,
"schedule": null,
"dosage_form": "Tablet, film coated",
"route": "Oral",
"application_number": "ANDA209288",
"generic": true,
"otc": false,
"approved": true,
"country": "US",
"mixture": false,
"allergenic": false,
"cosmetic": false,
"vaccine": false,
"ingredients": [
{
"drug": {
"drugbank_id": "DB01076",
"name": "Atorvastatin",
"cas_number": "134523-00-5",
"annotation_status": "complete",
"availability_by_region": [
{
"region": "ca",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
{
"region": "us",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
"..."
],
"description": "Atorvastatin (Lipitor®), is a lipid-lowering drug included in the statin class of medications...",
"simple_description": "A medication used to lower the amount of cholesterol in the blood and decrease the chance of having a heart attack or stroke.",
"clinical_description": "An HMG-CoA reductase inhibitor used to lower lipid levels and reduce the risk of cardiovascular disease including myocardial infarction and stroke.",
"synonyms": [
"Atorvastatin",
"atorvastatina",
"..."
],
"pharmacology": {
"indication_description": "Atorvastatin is indicated for the treatment of several types of dyslipidemias, including primary hyperlipidemia and mixed dyslipidemia in adults, hyper...",
"pharmacodynamic_description": "Atorvastatin is an oral antilipemic agent that reversibly inhibits HMG-CoA reductase...",
"mechanism_of_action_description": "Atorvastatin is a statin medication and a competitive inhibitor of the enzyme HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase, which catalyze...",
"absorption": "Atorvastatin presents a dose-dependent and non-linear pharmacokinetic profile...",
"protein_binding": "Atorvastatin is highly bound to plasma proteins and over 98% of the administered dose is found in a bound form.[F4670,F4673]",
"volume_of_distribution": [
"The reported volume of distribution of atorvastatin is of 380 L.[F4670,F4673]"
],
"clearance": [
"The registered total plasma clearance of atorvastatin is of 625 ml/min.[A19474]"
],
"half_life": "The half-life of atorvastatin is 14 hours while the half-life of its metabolites can reach up to 30 hours.[F4670,F4673]",
"route_of_elimination": "Atorvastatin and its metabolites are mainly eliminated in the bile without enterohepatic recirculation...",
"toxicity_description": "The reported LD50 of oral atorvastatin in mice is higher than 5000 mg/kg..."
},
"food_interactions": [
"Avoid grapefruit products. Grapefruit products may increase the risk for atorvastatin related adverse effects such as myopathy and rhabdomyolysis.",
"Take with or without food. Food decreases absorption but not to a clinically significant extent."
],
"identifiers": {
"drugbank_id": "DB01076",
"inchi": "InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-...",
"inchikey": "XUKUURHRXDUEBC-KAYWLYCHSA-N",
"atc_codes": [
{
"code": "C10BA05",
"title": "atorvastatin and ezetimibe",
"combination": true
},
{
"code": "C10BX11",
"title": "atorvastatin, amlodipine and perindopril",
"combination": true
},
"..."
]
},
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000391",
"name": "Hydroxymethylglutaryl-CoA Reductase Inhibitors",
"mesh_id": "D019161",
"mesh_tree_numbers": [
"D27.505.519.186.071.202.370",
"D27.505.519.389.370",
"..."
],
"atc_code": "C10AA",
"atc_level": 4,
"synonyms": [
"HMG Co A Reductase Inhibitors",
"HMG Co A Statins",
"..."
],
"description": "Compounds that inhibit HMG-CoA reductases. They have been shown to directly lower cholesterol synthesis."
},
{
"drugbank_id": "DBCAT005158",
"name": "Hypolipidemic Agents Indicated for Hyperlipidemia",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Lipid-Regulating Agents Indicated for Dyslipidemia"
],
"description": "Agents that have been assigned indications for the lowering of serum lipid levels..."
}
]
},
"strength": {
"number": "20",
"unit": "mg/1"
}
}
],
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000391",
"name": "Hydroxymethylglutaryl-CoA Reductase Inhibitors",
"mesh_id": "D019161",
"mesh_tree_numbers": [
"D27.505.519.186.071.202.370",
"D27.505.519.389.370",
"..."
],
"atc_code": "C10AA",
"atc_level": 4,
"synonyms": [
"HMG Co A Reductase Inhibitors",
"HMG Co A Statins",
"..."
],
"description": "Compounds that inhibit HMG-CoA reductases. They have been shown to directly lower cholesterol synthesis."
},
{
"drugbank_id": "DBCAT005158",
"name": "Hypolipidemic Agents Indicated for Hyperlipidemia",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Lipid-Regulating Agents Indicated for Dyslipidemia"
],
"description": "Agents that have been assigned indications for the lowering of serum lipid levels..."
}
],
"labeller": {
"name": "A-S Medication Solutions"
},
"images": []
}
This endpoint retrieves a specific drug product based on LOCAL PRODUCT ID corresponding to your region.
HTTP Request
Get https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID. |
Query Parameters
See Common Parameters.
Get a specific regional product for multi-region subscribers
Get a specific US product
curl -L 'https://api.drugbank.com/v1/products/50090-5181?region=us'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"ndc_product_code": "50090-5181",
"originator_ndc_product_code": "50090-5181",
"dpd_id": null,
"ema_product_code": null,
"ema_ma_number": null,
"name": "Atorvastatin Calcium",
"prescribable_name": "Atorvastatin calcium trihydrate 20 mg Oral Tablet",
"rx_norm_prescribable_name": "atorvastatin calcium 20 MG Oral Tablet",
"started_marketing_on": "2018-12-23",
"ended_marketing_on": null,
"approved_on": null,
"schedule": null,
"dosage_form": "Tablet, film coated",
"route": "Oral",
"application_number": "ANDA209288",
"generic": true,
"otc": false,
"approved": true,
"country": "US",
"mixture": false,
"allergenic": false,
"cosmetic": false,
"vaccine": false,
"ingredients": [
{
"name": "Atorvastatin",
"drugbank_id": "DB01076",
"strength": {
"number": "20",
"unit": "mg/1"
}
}
],
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000391",
"name": "Hydroxymethylglutaryl-CoA Reductase Inhibitors",
"mesh_id": "D019161",
"mesh_tree_numbers": [
"D27.505.519.186.071.202.370",
"D27.505.519.389.370",
"..."
],
"atc_code": "C10AA",
"atc_level": 4,
"synonyms": [
"HMG Co A Reductase Inhibitors",
"HMG Co A Statins",
"..."
],
"description": "Compounds that inhibit HMG-CoA reductases. They have been shown to directly lower cholesterol synthesis."
},
{
"drugbank_id": "DBCAT005158",
"name": "Hypolipidemic Agents Indicated for Hyperlipidemia",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Lipid-Regulating Agents Indicated for Dyslipidemia"
],
"description": "Agents that have been assigned indications for the lowering of serum lipid levels..."
}
],
"labeller": {
"name": "A-S Medication Solutions"
},
"images": []
}
HTTP Request
Get https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>?region=<REGION_CODE>
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| region | all subscribed regions | MUST be set to the region that corresponds to the Local Product ID |
Get a list of E.U. products by product code
curl -L 'https://api.drugbank.com/v1/eu/products/EMEA/H/C/000287'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"ndc_product_code": null,
"originator_ndc_product_code": null,
"dpd_id": null,
"ema_product_code": "EMEA/H/C/000287",
"ema_ma_number": "EU/1/99/125/001",
"name": "Zyprexa Velotab",
"prescribable_name": "Zyprexa 5 mg Disintegrating Oral Tablet",
"started_marketing_on": "2016-09-08",
"ended_marketing_on": null,
"approved_on": null,
"schedule": null,
"dosage_form": "Tablet, orally disintegrating",
"route": "Oral",
"application_number": null,
"generic": false,
"otc": false,
"approved": true,
"country": "EU",
"mixture": false,
"allergenic": false,
"cosmetic": false,
"vaccine": false,
"ingredients": [
{
"name": "Olanzapine",
"drugbank_id": "DB00334",
"strength": {
"number": "5",
"unit": "mg"
}
}
],
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000529",
"name": "Antipsychotic Agents",
"mesh_id": "D014150",
"mesh_tree_numbers": [
"D27.505.696.277.950.040",
"D27.505.954.427.210.950.040",
"..."
],
"atc_code": "N05A",
"atc_level": 3,
"synonyms": [
"Agents, Antipsychotic",
"Agents, Major Tranquilizing",
"..."
],
"description": "Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect..."
},
{
"drugbank_id": "DBCAT002673",
"name": "Antipsychotic Agents (Second Generation [Atypical])",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Atypical Antipsychotic",
"Atypical Antipsychotic Agents",
"..."
],
"description": null
}
],
"labeller": {
"name": "Eli Lilly Nederland B.V."
}
}
]
This endpoint retrieves a list of drug products based on EMA Product ID (European Medicines Agency ID). These products will have mostly the same information, although route, form and strengths may vary.
HTTP Request
GET https://api.drugbank.com/v1/eu/products/<EMA_ID>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The EMA ID of the product to retrieve. |
Query Parameters
See Common Parameters.
Get categories for a product
curl -L 'https://api.drugbank.com/v1/products/50090-5181/categories'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DBCAT003935",
"name": "Agents Causing Muscle Toxicity",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"categorization_kind": "indexing",
"synonyms": [
"Agents causing myopathy",
"Agents causing rhabdomyolysis",
"..."
],
"description": "Agents that cause muscle disorders including significant elevations of serum creatine kinase (CK), myopathy, myalgia, myositis, and rhabdomyolysis."
}
]
Returns an array of categories for a product, based on the Local Product ID of your region. See Regional Products.
The category type atc or mesh can be appended on to the end of the url to specify which hierarchy to retrieve the categories from. If left blank, DrugBank categories will be returned.
This endpoint supports pagination.
See Common Category Query Parameter Values for query parameters that affect the results of this request.
Shows all products for the drug regardless of the value of source, but parent/child relationships will be limited based on source.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/categories/<hierarchy (optional)>
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the categories. |
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | The Local Product ID of the product to retrieve the categories. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| categorization_kind | The categorization kind to filter by (optional). |
Get product concepts linked with a product
curl -L 'https://api.drugbank.com/v1/products/50090-5181/product_concepts'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Atorvastatin 20 mg",
"display_name": null,
"drugbank_pcid": "DBPC0031544",
"brand": null,
"level": 2,
"route": null,
"form": null,
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1996-12-17",
"regions": {
"us": true,
"canada": true,
"eu": false,
"germany": true,
"colombia": true,
"turkey": true,
"italy": true,
"malaysia": true,
"austria": true,
"indonesia": true,
"thailand": true,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Atorvastatin",
"drug": {
"name": "Atorvastatin",
"drugbank_id": "DB01076"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
}
]
This endpoint retrieves a list of product concepts linked to a product, based on the Local Product ID of your region. See Regional Products. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/product_concepts
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked product concepts. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| level | The product concept level to filter by (optional). | |
| min_level | The minimum product concept level to return (optional). | |
| min_level | The maximum product concept level to return (optional). | |
| unbranded_only | false | If true, returns only product concepts without an associated brand. |
Get indications linked with a product
curl -L 'https://api.drugbank.com/v1/products/50090-5181/indications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"kind": "used_as_adjunct_in_combination_to_manage",
"off_label": false,
"otc_use": false,
"adjunct_use": true,
"route": [
"Subcutaneous"
],
"dose_form": [
"Injection"
],
"dose_strength": [],
"combination_type": "regimen",
"age_groups": [
"adult"
],
"drug": {
"name": "Inclisiran",
"drugbank_id": "DB14901"
},
"regions": "US",
"min_age": {
"amount": 18,
"unit": "year"
},
"condition": {
"name": "Atherosclerotic Cardiovascular Diseases",
"drugbank_id": "DBCOND0078488",
"meddra_id": "llt/10051615",
"snomed_id": "d/300423010",
"icd10_id": "c/I25.1"
},
"with_therapies": [
{
"name": "Diet",
"drugbank_id": "DBCOND0034841",
"snomed_id": "d/493104010",
"icd10_id": "c/Z71.3"
}
],
"combination_drug_categories": [
{
"name": "Hydroxymethylglutaryl-CoA Reductase Inhibitors",
"drugbank_id": "DBCAT000391",
"mesh_id": "D019161"
}
]
}
]
This endpoint retrieves a list of indications linked to a product, based on the Local Product ID of your region. See Regional Products. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/indications
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked indications. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| off_label | null | Limits results by the value of the off_label attribute of the indications. |
| otc_use | null | Limits results by the value of the otc_use attribute of the indications. |
| adjunct_use | null | Limits results by the value of the adjunct_use attribute of the indications. |
| kind | null | Limits results by the value of the kind attribute of the indications. |
Get product concepts with similar indications
curl -L 'https://api.drugbank.com/v1/products/50090-5181/product_concepts/similar_indications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"similar_indications": [
{
"product_concept": {
"name": "Dapagliflozin Oral Tablet, film coated",
"display_name": null,
"drugbank_pcid": "DBPC0000198",
"brand": null,
"level": 3,
"route": "Oral",
"form": "Tablet, film coated",
"strengths": null,
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2008-01-14",
"regions": {
"us": true,
"canada": false,
"eu": true,
"germany": false,
"colombia": false,
"turkey": true,
"italy": true,
"malaysia": true,
"austria": true,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": false
},
"rxnorm_concepts": [
{
"name": "dapagliflozin Oral Tablet",
"RXCUI": "1488568"
}
],
"ingredients": [
{
"name": "Dapagliflozin",
"drug": {
"name": "Dapagliflozin",
"drugbank_id": "DB06292"
}
}
]
},
"from": {
"concept": {
"title": "Hospitalizations",
"drugbank_id": "DBCOND0105072"
},
"indication": {
"kind": "adjunct_therapy_in_prevention_of",
"off_label": false,
"otc_use": false,
"route": [
"Oral"
],
"dose_form": [
"Capsule"
],
"dose_strength": [],
"age_groups": [
"adult"
],
"drug": {
"name": "Icosapent ethyl",
"drugbank_id": "DB08887"
},
"regions": "US",
"condition": {
"name": "Hospitalizations",
"drugbank_id": "DBCOND0105072",
"meddra_id": "llt/10054112",
"snomed_id": "c/394656005"
},
"condition_associated_with": [
{
"name": "Cardiovascular Events",
"drugbank_id": "DBCOND0048671",
"snomed_id": "d/2157405016",
"icd10_id": "c/I24.9"
}
],
"patient_characteristics": [
{
"name": "Diabetes Mellitus",
"drugbank_id": "DBCOND0027886",
"meddra_id": "llt/10012601",
"snomed_id": "d/121589010"
},
{
"name": "CVD Risk Factors",
"drugbank_id": "DBCOND0076881",
"meddra_id": "llt/10078955",
"icd10_id": "c/Z91.89"
},
"..."
],
"adjunct_drug_categories": [
{
"name": "Hydroxymethylglutaryl-CoA Reductase Inhibitors",
"drugbank_id": "DBCAT000391",
"mesh_id": "D019161"
}
]
}
},
"to": {
"concept": {
"title": "Hospitalizations",
"drugbank_id": "DBCOND0105072"
},
"indication": {
"kind": "prevention_of",
"off_label": false,
"otc_use": false,
"route": [
"Oral"
],
"dose_form": [
"Tablet"
],
"dose_strength": [],
"age_groups": [
"adult"
],
"drug": {
"name": "Dapagliflozin",
"drugbank_id": "DB06292"
},
"regions": "US",
"condition": {
"name": "Hospitalizations",
"drugbank_id": "DBCOND0105072",
"meddra_id": "llt/10054112",
"snomed_id": "c/394656005"
},
"condition_associated_with": [
{
"name": "Heart Failure",
"drugbank_id": "DBCOND0027920",
"meddra_id": "llt/10019285",
"snomed_id": "d/2969213019",
"icd10_id": "c/I50.9"
}
],
"patient_characteristics": [
{
"name": "Type 2 Diabetes Mellitus",
"drugbank_id": "DBCOND0029752",
"meddra_id": "llt/10045242",
"snomed_id": "d/292566010",
"icd10_id": "c/E11"
},
{
"name": "Established cardiovascular disease",
"drugbank_id": "DBCOND0106958",
"icd10_id": "c/I51.9"
}
]
}
}
},
{
"product_concept": {
"name": "Dapagliflozin Oral Tablet",
"drugbank_pcid": "DBPC0132646",
"level": 3,
"route": "Oral",
"form": "Tablet",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2013-03-23",
"regions": {
"us": false,
"canada": true,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": false,
"mexico": true
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Dapagliflozin",
"drug": {
"name": "Dapagliflozin",
"drugbank_id": "DB06292"
}
}
]
},
"from": {
"concept": {
"title": "Hospitalizations",
"drugbank_id": "DBCOND0105072"
},
"indication": {
"kind": "adjunct_therapy_in_prevention_of",
"off_label": false,
"otc_use": false,
"route": [
"Oral"
],
"dose_form": [
"Capsule"
],
"dose_strength": [],
"age_groups": [
"adult"
],
"drug": {
"name": "Icosapent ethyl",
"drugbank_id": "DB08887"
},
"regions": "US",
"condition": {
"name": "Hospitalizations",
"drugbank_id": "DBCOND0105072",
"meddra_id": "llt/10054112",
"snomed_id": "c/394656005"
},
"condition_associated_with": [
{
"name": "Cardiovascular Events",
"drugbank_id": "DBCOND0048671",
"snomed_id": "d/2157405016",
"icd10_id": "c/I24.9"
}
],
"patient_characteristics": [
{
"name": "Diabetes Mellitus",
"drugbank_id": "DBCOND0027886",
"meddra_id": "llt/10012601",
"snomed_id": "d/121589010"
},
{
"name": "CVD Risk Factors",
"drugbank_id": "DBCOND0076881",
"meddra_id": "llt/10078955",
"icd10_id": "c/Z91.89"
},
"..."
],
"adjunct_drug_categories": [
{
"name": "Hydroxymethylglutaryl-CoA Reductase Inhibitors",
"drugbank_id": "DBCAT000391",
"mesh_id": "D019161"
}
]
}
},
"to": {
"concept": {
"title": "Hospitalizations",
"drugbank_id": "DBCOND0105072"
},
"indication": {
"kind": "prevention_of",
"off_label": false,
"otc_use": false,
"route": [
"Oral"
],
"dose_form": [
"Tablet"
],
"dose_strength": [],
"age_groups": [
"adult"
],
"drug": {
"name": "Dapagliflozin",
"drugbank_id": "DB06292"
},
"regions": "US",
"condition": {
"name": "Hospitalizations",
"drugbank_id": "DBCOND0105072",
"meddra_id": "llt/10054112",
"snomed_id": "c/394656005"
},
"condition_associated_with": [
{
"name": "Heart Failure",
"drugbank_id": "DBCOND0027920",
"meddra_id": "llt/10019285",
"snomed_id": "d/2969213019",
"icd10_id": "c/I50.9"
}
],
"patient_characteristics": [
{
"name": "Type 2 Diabetes Mellitus",
"drugbank_id": "DBCOND0029752",
"meddra_id": "llt/10045242",
"snomed_id": "d/292566010",
"icd10_id": "c/E11"
},
{
"name": "Established cardiovascular disease",
"drugbank_id": "DBCOND0106958",
"icd10_id": "c/I51.9"
}
]
}
}
},
"..."
]
}
This endpoint retrieves a list of product concepts which have indications similar to the indications of the provided product.
The results are returned as a JSON object with a “similar_indications” property containing an array of objects representing similar product concepts. Each object in this array has three properties: product_concept (the similar product_concept), from, and to.
From and to describe the relationship between the input product concept and the similar product concept. From and to both contain the same two properties:
- concept, a condition object, and
- indication, an indication object.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/product_concepts/similar_indications
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked product concepts with similar indications. |
Get drug-drug interactions for a product
curl -L 'https://api.drugbank.com/v1/products/17856-0166/ddi?severity=moderate&available_only=true'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"product_ingredient": {
"drugbank_id": "DB00316",
"name": "Acetaminophen"
},
"affected_product_ingredient": {
"drugbank_id": "DB01281",
"name": "Abatacept"
},
"description": "The metabolism of Acetaminophen can be increased when combined with Abatacept.",
"extended_description": "The formation of CYP450 enzymes is inhibited by the presence of increased levels of cytokines during chronic inflammation...",
"action": "increase_dynamics",
"severity": "moderate",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_1",
"management": "When treatment with agents that reduce cytokine levels is initiated or discontinued, monitor for reduced efficacy of drugs that are CYP1A2 substrates a..."
}
]
This endpoint retrieves a list of drug-drug interactions linked to a product, based on the Local Product ID of your region. See Regional Products. The product_ingredient refers to the interacting ingredient in the specified product, while the affected_product_ingredient is the drug it interacts with. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/ddi
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked interactions. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| severity | null | Limits results by the severity of the interactions. May be major, moderate, or minor. |
| evidence_level | null | Limits results by the evidence level of the interactions. May be level_1 or level_2. |
| available_only | false | If true, only includes interactions with currently available drugs. If a region is specified, the drugs must also be available in that region. |
| include_references | false | If true, includes the references for each interaction. See References for details on the format. |
Get adverse effects linked with a product
curl -L 'https://api.drugbank.com/v1/products/50580-0576/adverse_effects'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
},
"route": [
"Intravenous"
],
"dose_form": [],
"evidence_type": [
"clinical_trial"
],
"admin": "multiple dose",
"regions": "US",
"age_groups": [
"adult"
],
"incidences": [
{
"kind": "experimental",
"percent": "34%"
},
{
"kind": "placebo",
"percent": "31%"
}
],
"effect": {
"name": "Nausea",
"drugbank_id": "DBCOND0010699",
"meddra_id": "pt/10028813",
"snomed_id": "d/122688013",
"icd10_id": "c/R11.0"
}
}
]
This endpoint retrieves a list of adverse effects linked to a product, based on the Local Product ID of your region. See Regional Products. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/adverse_effects
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked adverse effects. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
include_references |
false | If true, includes the list of references for each adverse effect associated with this product. See References for details. |
Get contraindications linked with a product
curl -L 'https://api.drugbank.com/v1/products/16590-0022/contraindications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Caffeine",
"drugbank_id": "DB00201"
},
"route": [
"Oral"
],
"dose_form": [],
"hypersensitivity": [
"true"
],
"lab_values": [],
"recommended_actions": [],
"regions": "US"
}
]
This endpoint retrieves a list of contraindications linked to a product, based on the Local Product ID of your region. See Regional Products. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/contraindications
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked contraindications. |
Get boxed warnings linked with a product
curl -L 'https://api.drugbank.com/v1/products/16590-0022/boxed_warnings'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Caffeine",
"drugbank_id": "DB00201"
},
"kind": "warning",
"lab_values": [],
"route": [],
"dose_form": [],
"risk": {
"name": "Cerebral Ischemia",
"drugbank_id": "DBCOND0031659",
"meddra_id": "llt/10008120",
"snomed_id": "d/427284014",
"icd10_id": "c/I67.82"
},
"with_drugs": [
{
"name": "Ergotamine",
"drugbank_id": "DB00696"
}
],
"with_categories": [
{
"name": "Cytochrome P-450 CYP3A4 Inhibitors (strong)",
"drugbank_id": "DBCAT002647"
}
]
}
]
This endpoint retrieves a list of boxed warnings (Black Box warnings) linked to a product, based on the Local Product ID of your region. See Regional Products. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/boxed_warnings
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked boxed_warnings. |
Get packages for a product
curl -L 'https://api.drugbank.com/v1/products/63304-803/packages'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"package_ndc_code": "63304-0803-30",
"originator_package_ndc_code": "63304-803-30",
"description": "30 TABLET IN 1 BOTTLE",
"full_description": "30 TABLET IN 1 BOTTLE",
"amount": "30",
"unit": "1",
"form": "BOTTLE"
},
{
"package_ndc_code": "63304-0803-05",
"originator_package_ndc_code": "63304-803-05",
"description": "500 TABLET IN 1 BOTTLE",
"full_description": "500 TABLET IN 1 BOTTLE",
"amount": "500",
"unit": "1",
"form": "BOTTLE"
}
]
This endpoint retrieves a list of top level packages for a product, based on the Local Product ID of your region. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/packages
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the packages. |
Get the most current FDA label for a product
curl -L 'https://api.drugbank.com/v1/products/50580-111/label'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"id": "5a42290a-810c-427c-a406-2df70a02c372",
"set_id": "81f3847f-99e8-4ff3-ad18-f8b8e1c54238",
"version": 1,
"current": true,
"title": "Tylenol Extra Strength - Acetaminophen LIQUID",
"product_type": "HUMAN OTC DRUG LABEL",
"effective_on": "2012-12-10",
"labeler_name": "McNeil Consumer Healthcare Div. McNeil-PPC, Inc",
"drugbank_status": "processed",
"first_product_marketing_started_on": "1998-04-01",
"last_product_marketing_ended_on": "2012-10-31",
"pmg_section_count": 6,
"hpi_section_count": 10,
"sli_section_count": 2,
"all_section_count": 17,
"abuse": null,
"accessories": null,
"active_ingredient": "<section id=\"section-2\" class=\"content-section\" data-section-code=\"55106-9\"><h1>Active ingredient (in each 15 mL = 1 tablespoon)</h1>\n<div class=\"conte...",
"adverse_reactions": null,
"alarms": null,
"animal_pharmacology_and_or_toxicology": null,
"ask_doctor": "<section id=\"section-5...",
"ask_doctor_or_pharmacist": "<section id=\"section-5...",
"assembly_or_installation_instructions": null,
"boxed_warning": null,
"calibration_instructions": null,
"carcinogenesis_and_mutagenesis_and_impairment_of_fertility": null,
"cleaning": null,
"clinical_pharmacology": null,
"clinical_studies": null,
"compatible_accessories": null,
"components": null,
"contraindications": null,
"controlled_substance": null,
"dependence": null,
"description": null,
"diagram_of_device": null,
"disposal_and_waste_handling": null,
"do_not_use": "<section id=\"section-5...",
"dosage_and_administration": "<section id=\"section-6\" class=\"content-section\" data-section-code=\"34068-7\"><h1>Directions</h1>\n<div class=\"content-section-inner\" id=\"\">\n<ul class=\"sq...",
"dosage_forms_and_strengths": null,
"drug_abuse_and_dependence": null,
"drug_interactions": null,
"drug_and_or_laboratory_test_interactions": null,
"environmental_warning": null,
"food_safety_warning": null,
"general_precautions": null,
"geriatric_use": null,
"guaranteed_analysis_of_feed": null,
"health_care_provider_letter": null,
"health_claim": null,
"how_supplied": null,
"inactive_ingredient": "<section id=\"section-8\" class=\"content-section\" data-section-code=\"51727-6\"><h1>Inactive ingredients</h1>\n<div class=\"content-section-inner\" id=\"\"><p>a...",
"indications_and_usage": "<section id=\"section-4\" class=\"content-section\" data-section-code=\"34067-9\"><h1>Uses</h1>\n<div class=\"content-section-inner\" id=\"\"><ul class=\"square\">\n...",
"information_for_owners_or_caregivers": null,
"information_for_patients": null,
"instructions_for_use": null,
"intended_use_of_the_device": null,
"keep_out_of_reach_of_children": "<section id=\"section-5...",
"labor_and_delivery": null,
"laboratory_tests": null,
"mechanism_of_action": null,
"microbiology": null,
"nonclinical_toxicology": null,
"nonteratogenic_effects": null,
"nursing_mothers": null,
"other_safety_information": null,
"overdosage": null,
"package_label_principal_display_panel": "<section id=\"section-10\" class=\"content-section\" data-section-code=\"51945-4\"><h1>PRINCIPAL DISPLAY PANEL</h1>\n<div class=\"content-section-inner\" id=\"\">...",
"patient_medication_information": null,
"pediatric_use": null,
"pharmacodynamics": null,
"pharmacogenomics": null,
"pharmacokinetics": null,
"precautions": null,
"pregnancy": null,
"pregnancy_or_breast_feeding": "<section id=\"section-5...",
"purpose": "<section id=\"section-3\" class=\"content-section\" data-section-code=\"55105-1\"><h1>Purpose</h1>\n<div class=\"content-section-inner\" id=\"\"><p>Pain reliever/...",
"questions": "<section id=\"section-9\" class=\"content-section\" data-section-code=\"53413-1\"><h1>Questions or comments?</h1>\n<div class=\"content-section-inner\" id=\"\"><p...",
"recent_major_changes": null,
"references": null,
"residue_warning": null,
"risks": null,
"route": null,
"safe_handling_warning": null,
"spl_indexing_data_elements": null,
"spl_medguide": null,
"spl_patient_package_insert": null,
"spl_product_data_elements": null,
"spl_unclassified": "<section id=\"section-1\" class=\"content-section\" data-section-code=\"42229-5\"><div class=\"content-section-inner\" id=\"\"><p><span class=\"bold italics\">Drug...",
"statement_of_identity": null,
"stop_use": "<section id=\"section-5...",
"storage_and_handling": "<section id=\"section-7\" class=\"content-section\" data-section-code=\"44425-7\"><h1>Other information</h1>\n<div class=\"content-section-inner\" id=\"\"><ul cla...",
"summary_of_safety_and_effectiveness": null,
"teratogenic_effects": null,
"troubleshooting": null,
"use_in_specific_populations": null,
"user_safety_warnings": null,
"warnings": "<section id=\"section-5\" class=\"content-section\" data-section-code=\"34071-1\"><h1>Warnings</h1>\n<div class=\"content-section-inner\" id=\"\">\n<section id=\"se...",
"warnings_and_cautions": null,
"when_using": null
}
This endpoint retrieves the most current FDA label for a drug product, based on NDC ID.
HTTP Request
GET https://api.drugbank.com/v1/products/<NDC_ID>/label
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| content_format | html | Format to return for section content from label, either html (default) or text. |
View Drug Alternatives for a Product
curl -L 'https://api.drugbank.com/v1/products/57841-1150/product_concepts/therapeutic_alternatives'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Omeprazole",
"drugbank_id": "DB00338",
"categories": [
{
"drugbank_id": "DBCAT000541",
"name": "Proton Pump Inhibitors",
"mesh_id": "D054328",
"mesh_tree_numbers": [
"D27.505.519.389.848"
],
"atc_code": "A02BC",
"atc_level": 4,
"categorization_kinds": [
"therapeutic",
"pharmacological",
"..."
],
"synonyms": [
"Inhibitors, Proton Pump",
"PP Inhibitors",
"..."
],
"description": "Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE...",
"drugs": [
{
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
{
"name": "Lansoprazole",
"drugbank_id": "DB00448"
},
"..."
]
},
{
"drugbank_id": "DBCAT003638",
"name": "Proton-pump Inhibitors",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"categorization_kinds": [
"therapeutic"
],
"synonyms": [],
"description": null,
"drugs": [
{
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
{
"name": "Lansoprazole",
"drugbank_id": "DB00448"
},
"..."
]
}
]
}
]
This endpoint retrieves lists of drugs as therapeutic alternatives based on the therapeutic categorizations of the ingredients associated with the provided product.
Related products to the drug alternatives can then be found using this endpoint.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/product_concepts/therapeutic_ alternatives
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked therapeutic alternatives. |
Product Concepts
About Product Concepts
DrugBank ID | Title | Level | Ingredients? | Exact Ingredients? | Strength? | Route? | Form? | Brand? |
|---|---|---|---|---|---|---|---|---|
DBPC0017857 | Tamoxifen | 1 | yes | no | no | no | no | no |
DBPC0017859 | Tamoxifen 10 mg | 2 | yes | no | yes | no | no | no |
DBPC0017861 | Tamoxifen Oral | 2 | yes | no | no | yes | no | no |
DBPC0103411 | Tamoxifen Tablet | 2 | yes | no | no | no | yes | no |
DBPC0017858 | Tamoxifen citrate | 2 | yes | yes | no | no | no | no |
DBPC0017863 | Tamoxifen 10 mg Oral | 3 | yes | no | yes | yes | no | no |
DBPC0104223 | Tamoxifen 10 mg Tablet | 3 | yes | no | yes | no | yes | no |
DBPC0103415 | Tamoxifen Oral Tablet | 3 | yes | no | no | yes | yes | no |
DBPC0017860 | Tamoxifen citrate 10 mg | 3 | yes | yes | yes | no | no | no |
DBPC0017862 | Tamoxifen citrate Oral | 3 | yes | yes | no | yes | no | no |
DBPC0103412 | Tamoxifen citrate Tablet | 3 | yes | yes | no | no | yes | no |
DBPC0104225 | Tamoxifen 10 mg Oral Tablet | 4 | yes | no | yes | yes | yes | no |
DBPC0017864 | Tamoxifen citrate 10 mg Oral | 4 | yes | yes | yes | yes | no | no |
DBPC0104224 | Tamoxifen citrate 10 mg Tablet | 4 | yes | yes | yes | no | yes | no |
DBPC0103416 | Tamoxifen citrate Oral Tablet | 4 | yes | yes | no | yes | yes | no |
DBPC0104226 | Tamoxifen citrate 10 mg Oral Tablet | 5 | yes | yes | yes | yes | yes | no |
Product concepts enable you to flexibly represent and query drug products. With them, you can:
- use a stable vocabulary which abstracts over many equivalent or similar products
- easily navigate between related products, at varying and specifiable levels of similarity
- filter products at increasing levels of detail by taking advantage of the hierarchy of product concepts
The basis of product concepts is their many-to-many relationship to products. Each product has many product concepts, each of which match that product in a specific way. Each product concept can be shared between multiple products - if the products are similar enough. Each product concept describes a specific subset of the properties of the products it is matched to. The connection between product and product concept is derived directly from the properties that make up the two objects.
For instance, the US product with the NDC product code 51862-449 has 16 product concepts. These are listed in the table to the right. In this case, the product is not branded, so there are no product concepts with a brand. Each product concept offers a view into the product.
The value of the level property indicates how many fields are set in the product concept. The most general product concept, DBPC0017857 Tamoxifen will be shared between all products which contain Tamoxifen as their sole active ingredient. As the level increases, the product concept becomes more specific, and generally, it will match fewer products. Product concepts are derived from products, so there is a direct correspondance between the properties of the product concept and the properties of the products it is mapped to.
Product concepts can therefore be used as both a filtered version of a specific product (eg. specifying the ingredients and route, but not the form or strength), or as a search filter which can find all products matching a given criteria.
Finally, product concepts are connected in a tree structure. Each level 5 concept is a child of one or more level 4 concepts, which is a child of one or more level 3 concepts, and so on. A child concept adds one single field to its parent concepts. For instance, the level 4 DBPC0103416 Tamoxifen citrate Oral Tablet is a child of the level 3 concept DBPC0103412 Tamoxifen citrate Oral - it adds the form Tablet. It is also a child of the level 3 concept DBPC0103412 Tamoxifen citrate Tablet - to this parent it adds the route Oral.
Errors
The product concept API revolves around product concept objects. Thefore there are common error messages which may be returned from any of the product concept APIs, depending on the state of the product concept involved.
- API returns status code 404 if the product concept does not exist
- API returns status code 410 if the product concept has been revoked
Data Types
The product concepts API uses the following data types:
Product Concepts
{
"name": "Lopinavir 100 mg / Ritonavir 25 mg Oral Tablet, film coated [Kaletra]",
"display_name": "Kaletra 100 mg, 25 mg Oral Tablet, film coated",
"drugbank_pcid": "DBPC0198939",
"brand": "Kaletra",
"level": 5,
"route": "Oral",
"form": "Tablet, film coated",
"strengths": "100 mg, 25 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2006-12-21",
"regions": {
"us": true,
"canada": false,
"eu": false
},
"rxnorm_concepts": [
{
"name": "lopinavir 100 MG / Ritonavir 25 MG Oral Tablet [Kaletra]",
"RXCUI": "847745"
}
],
"ingredients": [
{
"name": "Lopinavir",
"drug": {
"name": "Lopinavir",
"drugbank_id": "DB01601"
},
"strength": {
"amount": "100.0",
"per": "1.0",
"units": "mg"
}
},
{
"name": "Ritonavir",
"drug": {
"name": "Ritonavir",
"drugbank_id": "DB00503"
},
"strength": {
"amount": "25.0",
"per": "1.0",
"units": "mg"
}
}
]
}
| Property | Type | Description |
|---|---|---|
| drugbank_pcid | string | DrugBank id for the product concept. |
| name | string | The name of this product concept. Name describes all key characteristics. |
| display_name | string | An optional, more compact name, based on the brand name. |
| level | int | Level of the product concept in the hierarchy. 0 = root. |
| standing | string | Indicates the level of connection this product concept has to products. |
| standing_updated_at | date | Indicates the date of the last update to the standing of the product concept |
| standing_active_since | date | Indicates the date that the standing became “active” |
| regions | object | Indicates the availability of this product concept the regions “us”, “canada”, and “eu” |
| brand | string | |
| form | string | |
| route | string | |
| strengths | string | A string representing the strengths of the ingredients |
| rxnorm_concepts | array of objects | An array of objects with name and RXCUI properties, indicating equivalent RxNorm concepts. |
| ingredients | array of objects | The ingredients in the product concept. |
Standings
The standing property can have the following values:
- active
- inactive
- archived
Product Concept Ingredients
{
"name": "Lopinavir",
"drug": {
"name": "Lopinavir",
"drugbank_id": "DB01601"
},
"strength": {
"amount": "100.0",
"per": "1.0",
"units": "mg"
}
}
| Property | Type | Description |
|---|---|---|
| drug | object | The drug being represented as an ingredient. Includes name and drugbank_id properties. |
| salt | object | The salt being represented as an ingredient. Includes name and drugbank_id properties. |
| amount | string | |
| per | string | |
| units | string |
Product Concepts Search
Searching for a specific product concept can be achieved using our medication name search endpoint.
View Product Concept
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0001930'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"name": "Pantoprazole sodium 20 mg Oral",
"display_name": null,
"drugbank_pcid": "DBPC0001930",
"brand": null,
"level": 4,
"route": "Oral",
"form": null,
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1999-01-29",
"regions": {
"us": true,
"canada": true,
"eu": false,
"germany": false,
"colombia": true,
"turkey": true,
"italy": false,
"malaysia": true,
"austria": true,
"indonesia": false,
"thailand": true,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole as Pantoprazole sodium",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"exact_ingredient": {
"name": "Pantoprazole sodium",
"drugbank_id": "DBSALT000386"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
}
This endpoint retrieves a specific product concept based on ID (DrugBank Product Concept ID).
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| include_simple_desc | false | If set to true, include simple descriptions for the product concept ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product concept ingredients. |
View Product Concept Products
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0001930/products'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"ndc_product_code": null,
"originator_ndc_product_code": null,
"dpd_id": null,
"ema_product_code": null,
"ema_ma_number": null,
"pzn": null,
"co_product_id": "INVIMA 2017M-0012470-R1",
"tr_product_id": null,
"it_product_id": null,
"my_product_id": null,
"at_product_id": null,
"idn_product_id": null,
"th_product_id": null,
"sg_product_id": null,
"mx_registration_id": null,
"mx_product_id": null,
"name": "PANTOPRAZOL 20 MG TABLETAS GASTRORESISTENTES",
"prescribable_name": "Pantoprazole sodium 20 mg Delayed Release Oral Tablet",
"started_marketing_on": "2011-03-30",
"ended_marketing_on": null,
"approved_on": null,
"schedule": null,
"dosage_form": "Tablet, delayed release",
"route": "Oral",
"application_number": null,
"generic": false,
"otc": false,
"approved": false,
"country": "Colombia",
"mixture": false,
"allergenic": false,
"cosmetic": false,
"vaccine": false,
"ingredients": [
{
"name": "Pantoprazole",
"drugbank_id": "DB00213",
"strength": {
"number": "20",
"unit": "mg"
}
}
],
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000541",
"name": "Proton Pump Inhibitors",
"mesh_id": "D054328",
"mesh_tree_numbers": [
"D27.505.519.389.848"
],
"atc_code": "A02BC",
"atc_level": 4,
"synonyms": [
"Inhibitors, Proton Pump",
"PP Inhibitors",
"..."
],
"description": "Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE..."
},
{
"drugbank_id": "DBCAT003638",
"name": "Proton-pump Inhibitors",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"synonyms": [],
"description": null
}
],
"labeller": {
"name": "GENVEON ILAC SANAYI VETICARET A.S."
}
}
]
This returns a list of products which are described by the product concept specified by ID (DrugBank Product Concept ID). These results are region-filtered, making it possible to find products from one or all regions by varying the region parameter.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/products
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| include_simple_desc | false | If set to true, include simple descriptions for the product ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product ingredients. |
Filters
Products can be narrowed down by appending a filter to the url in the following format:
https://api.drugbank.com/v1/product_concepts/<ID>/products/<filter>
For example:
https://api.drugbank.com/v1/product_concepts/<ID>/products/generics
| Filter | Description |
|---|---|
| generics | Returns only generic i.e. unbranded products. |
View Product Concept Routes
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0001930/routes'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Pantoprazole sodium 20 mg Oral Tablet, coated",
"display_name": null,
"drugbank_pcid": "DBPC0656321",
"brand": null,
"level": 5,
"route": "Oral",
"form": "Tablet, coated",
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": null,
"standing_active_since": "1999-10-07",
"regions": {
"us": false,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": true,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole as Pantoprazole sodium",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"exact_ingredient": {
"name": "Pantoprazole sodium",
"drugbank_id": "DBSALT000386"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
},
{
"name": "Pantoprazole sodium 20 mg Oral Tablet",
"display_name": null,
"drugbank_pcid": "DBPC0637413",
"brand": null,
"level": 5,
"route": "Oral",
"form": "Tablet",
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": null,
"standing_active_since": "2011-08-18",
"regions": {
"us": true,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": true,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole as Pantoprazole sodium",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"exact_ingredient": {
"name": "Pantoprazole sodium",
"drugbank_id": "DBSALT000386"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
},
"..."
]
This returns a list of product concepts which are more specific versions of the product concept specified by <ID>. Each of the returned product concepts has a route specified.
When using this endpoint, the "route" field of the returned product concepts can be displayed to users to communicate route separately from ingredients, brand, form, etc.
The returned routes can be further refined by passing in a full-text search parameter q. This query parameter works similarity to the medication name search and searches brand names, ingredient names and synonyms as well as ingredient names and synonyms. It also allows the query_type and hit_details parameters to be used.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/routes
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | The text-search query string (optional). | |
| query_type | simple | If set to advanced, allows basic boolean operations in the query parameter q. If set to exact, only returns product concepts containing an exact match to the query string q. Only applies if q is also given. |
| hit_details | false | If set to true, returns additional information on matched brands/ingredients/products for the concept. Only applies if q is also given. |
| withdrawn | false | If set to include, includes product concepts for which all products have been withdrawn. If set to true, includes only product concepts for which all products have been withdrawn. If set to false, includes only product concepts with at least one non-withdrawn product. |
| include_simple_desc | false | If set to true, include simple descriptions for the product concept ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product concept ingredients. |
View Product Concept Forms
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0001930/forms'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Pantoprazole sodium 20 mg Oral Tablet",
"display_name": null,
"drugbank_pcid": "DBPC0637413",
"brand": null,
"level": 5,
"route": "Oral",
"form": "Tablet",
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": null,
"standing_active_since": "2011-08-18",
"regions": {
"us": true,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": true,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole as Pantoprazole sodium",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"exact_ingredient": {
"name": "Pantoprazole sodium",
"drugbank_id": "DBSALT000386"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
},
{
"name": "Pantoprazole sodium 20 mg Oral Tablet, coated",
"display_name": null,
"drugbank_pcid": "DBPC0656321",
"brand": null,
"level": 5,
"route": "Oral",
"form": "Tablet, coated",
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": null,
"standing_active_since": "1999-10-07",
"regions": {
"us": false,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": true,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole as Pantoprazole sodium",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"exact_ingredient": {
"name": "Pantoprazole sodium",
"drugbank_id": "DBSALT000386"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
},
"..."
]
This returns a list of product concepts which are more specific versions of the product concept specified by <ID>. Each of the returned product concepts has a form specified.
When using this endpoint, the "form" field of the returned product concepts can be displayed to users to communicate form separately from ingredients, brand, route, etc.
The returned forms can be further refined by passing in a full-text search parameter q. This query parameter works similarity to the medication name search and searches brand names, ingredient names and synonyms as well as ingredient names and synonyms. It also allows the query_type and hit_details parameters to be used.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/forms
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | The text-search query string (optional). | |
| query_type | simple | If set to advanced, allows basic boolean operations in the query parameter q. If set to exact, only returns product concepts containing an exact match to the query string q. Only applies if q is also given. |
| hit_details | false | If set to true, returns additional information on matched brands/ingredients/products for the concept. Only applies if q is also given. |
| withdrawn | false | If set to include, includes product concepts for which all products have been withdrawn. If set to true, includes only product concepts for which all products have been withdrawn. If set to false, includes only product concepts with at least one non-withdrawn product. |
| include_simple_desc | false | If set to true, include simple descriptions for the product concept ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product concept ingredients. |
View Product Concept Strengths
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0001930/strengths'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Pantoprazole sodium 20 mg Oral Tablet, coated",
"display_name": null,
"drugbank_pcid": "DBPC0656321",
"brand": null,
"level": 5,
"route": "Oral",
"form": "Tablet, coated",
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": null,
"standing_active_since": "1999-10-07",
"regions": {
"us": false,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": true,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole as Pantoprazole sodium",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"exact_ingredient": {
"name": "Pantoprazole sodium",
"drugbank_id": "DBSALT000386"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
},
{
"name": "Pantoprazole sodium 20 mg Oral Tablet",
"display_name": null,
"drugbank_pcid": "DBPC0637413",
"brand": null,
"level": 5,
"route": "Oral",
"form": "Tablet",
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": null,
"standing_active_since": "2011-08-18",
"regions": {
"us": true,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": true,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole as Pantoprazole sodium",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"exact_ingredient": {
"name": "Pantoprazole sodium",
"drugbank_id": "DBSALT000386"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
},
"..."
]
This returns a list of product concepts which are more specific versions of the product concept specified by <ID>. Each of the returned product concepts has ingredient strengths specified.
When using this endpoint, the "strengths" field of the returned product concepts can be displayed to users to communicate strength separately from ingredients, brand, route, etc.
The returned strengths can be further refined by passing in a full-text search parameter q. This query parameter works similarity to the medication name search and searches brand names, ingredient names and synonyms as well as ingredient names and synonyms. It also allows the query_type and hit_details parameters to be used.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/strengths
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | The text-search query string (optional). | |
| query_type | simple | If set to advanced, allows basic boolean operations in the query parameter q. If set to exact, only returns product concepts containing an exact match to the query string q. Only applies if q is also given. |
| hit_details | false | If set to true, returns additional information on matched brands/ingredients/products for the concept. Only applies if q is also given. |
| withdrawn | false | If set to include, includes product concepts for which all products have been withdrawn. If set to true, includes only product concepts for which all products have been withdrawn. If set to false, includes only product concepts with at least one non-withdrawn product. |
| include_simple_desc | false | If set to true, include simple descriptions for the product concept ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product concept ingredients. |
View Product Concept Categories
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0001930/categories'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DBCAT000768",
"name": "2-Pyridinylmethylsulfinylbenzimidazoles",
"mesh_id": "D053799",
"mesh_tree_numbers": [
"D02.886.640.074",
"D03.383.725.024",
"..."
],
"atc_code": null,
"atc_level": null,
"categorization_kind": "indexing",
"synonyms": [
"2 Methylpyridine 2 Benzimidazole Sulfoxides",
"2 Pyridinylmethylsulfinyl 2 Benzimidazoles",
"..."
],
"description": "Compounds that contain benzimidazole joined to a 2-methylpyridine via a sulfoxide linkage..."
}
]
Returns an array of categories for the product concept specified by ID (DrugBank Product Concept ID).
The category type atc or mesh can be appended on to the end of the url to specify which hierarchy to retrieve the categories from. If left blank, DrugBank categories will be returned.
This endpoint supports pagination.
See Common Category Query Parameter Values for query parameters that affect the results of this request.
Shows all categories for the product concept regardless of the value of source, but parent/child relationships will be limited based on source.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/categories/<hierarchy (optional)>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked categories. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| categorization_kind | The categorization kind to filter by (optional). |
View Product Concept Indications
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0131467/indications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"kind": "treatment_of",
"off_label": false,
"otc_use": false,
"adjunct_use": false,
"route": [
"Intravenous",
"Subcutaneous"
],
"dose_form": [
"Injection"
],
"dose_strength": [],
"timeline": "When chemotherapy is planned to last ≥2 months",
"drug": {
"name": "Darbepoetin alfa",
"drugbank_id": "DB00012"
},
"regions": "US",
"condition": {
"name": "Anemia",
"drugbank_id": "DBCOND0020261",
"meddra_id": "llt/10002034",
"snomed_id": "d/535044014",
"icd10_id": "c/D64.9"
},
"condition_associated_with": [
{
"name": "Myelosuppressive chemotherapy",
"drugbank_id": "DBCOND0020351"
}
]
}
]
This returns a list of indications linked to the product concept specified by ID (DrugBank Product Concept ID). This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/indications
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| off_label | null | Limits results by the value of the off_label attribute of the indications. |
| otc_use | null | Limits results by the value of the otc_use attribute of the indications. |
| adjunct_use | null | Limits results by the value of the adjunct_use attribute of the indications. |
| kind | null | Limits results by the value of the kind attribute of the indications. |
View product concepts with similar indications
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0131467/similar_indications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"similar_indications": [
{
"product_concept": {
"name": "Luspatercept Subcutaneous",
"display_name": null,
"drugbank_pcid": "DBPC0493704",
"brand": null,
"level": 2,
"route": "Subcutaneous",
"form": null,
"strengths": null,
"standing": "active",
"standing_updated_at": null,
"standing_active_since": "2019-11-08",
"regions": {
"us": true,
"canada": true,
"eu": true,
"germany": false,
"colombia": true,
"turkey": false,
"italy": true,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": false,
"mexico": true
},
"rxnorm_concepts": [
{
"name": "luspatercept Injection",
"RXCUI": "2262905"
},
{
"name": "luspatercept Injectable Product",
"RXCUI": "2262904"
}
],
"ingredients": [
{
"name": "Luspatercept",
"drug": {
"name": "Luspatercept",
"drugbank_id": "DB12281"
}
}
]
},
"from": {
"concept": {
"title": "Anemia",
"drugbank_id": "DBCOND0020261"
},
"indication": {
"kind": "treatment_of",
"off_label": false,
"otc_use": false,
"route": [
"Intravenous",
"Subcutaneous"
],
"dose_form": [
"Injection"
],
"dose_strength": [],
"timeline": "When chemotherapy is planned to last ≥2 months",
"drug": {
"name": "Darbepoetin alfa",
"drugbank_id": "DB00012"
},
"regions": "US",
"condition": {
"name": "Anemia",
"drugbank_id": "DBCOND0020261",
"meddra_id": "llt/10002034",
"snomed_id": "d/535044014",
"icd10_id": "c/D64.9"
},
"condition_associated_with": [
{
"name": "Myelosuppressive chemotherapy",
"drugbank_id": "DBCOND0020351"
}
]
}
},
"to": {
"concept": {
"title": "Anemia",
"drugbank_id": "DBCOND0020261"
},
"indication": {
"kind": "treatment_of",
"off_label": false,
"otc_use": false,
"route": [
"Subcutaneous"
],
"dose_form": [],
"dose_strength": [],
"timeline": "following failure of erythropoiesis stimulating agent treatment",
"age_groups": [
"adult"
],
"drug": {
"name": "Luspatercept",
"drugbank_id": "DB12281"
},
"regions": "US",
"condition": {
"name": "Anemia",
"drugbank_id": "DBCOND0020261",
"meddra_id": "llt/10002034",
"snomed_id": "d/535044014",
"icd10_id": "c/D64.9"
},
"patient_characteristics": [
{
"name": "Require regular red blood cell transfusions",
"drugbank_id": "DBCOND0124075"
},
{
"name": "Myelodysplastic/Myeloproliferative Neoplasm With Ring Sideroblasts and Thrombocytosis",
"drugbank_id": "DBCOND0118093",
"snomed_id": "d/4652416018"
}
]
}
}
},
{
"product_concept": {
"name": "Capecitabine Oral Tablet",
"drugbank_pcid": "DBPC0124353",
"level": 3,
"route": "Oral",
"form": "Tablet",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1998-01-01",
"regions": {
"us": true,
"canada": true,
"eu": true,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [
{
"name": "capecitabine Oral Tablet",
"RXCUI": "371250"
}
],
"ingredients": [
{
"name": "Capecitabine",
"drug": {
"name": "Capecitabine",
"drugbank_id": "DB01101"
}
}
]
},
"from": {
"concept": {
"title": "myelosuppressive chemotherapy",
"drugbank_id": "DBCOND0020351"
},
"indication": {
"kind": "treatment_of",
"off_label": false,
"otc_use": false,
"route": [
"Intravenous",
"Subcutaneous"
],
"dose_form": [
"Injection"
],
"dose_strength": [],
"timeline": "When chemotherapy is planned to last ≥2 months",
"drug": {
"name": "Darbepoetin alfa",
"drugbank_id": "DB00012"
},
"regions": "US",
"condition": {
"name": "Anemia",
"drugbank_id": "DBCOND0020261",
"meddra_id": "llt/10002034",
"snomed_id": "d/535044014",
"icd10_id": "c/D64.9"
},
"condition_associated_with": [
{
"name": "Myelosuppressive chemotherapy",
"drugbank_id": "DBCOND0020351"
}
]
}
},
"to": {
"concept": {
"title": "Chemotherapy",
"drugbank_id": "DBCOND0020513"
},
"indication": {
"kind": "for_therapy",
"off_label": false,
"otc_use": false,
"route": [
"Oral"
],
"dose_form": [
"Tablet"
],
"dose_strength": [],
"age_groups": [
"adult"
],
"drug": {
"name": "Capecitabine",
"drugbank_id": "DB01101"
},
"regions": "US",
"condition": {
"name": "Metastatic Adenocarcinoma of the Gastroesophageal Junction",
"drugbank_id": "DBCOND0069248",
"modification_of": {
"condition_stage": "metastatic",
"base": {
"name": "Adenocarcinomas of the Gastroesophageal Junction",
"drugbank_id": "DBCOND0032762",
"meddra_id": "llt/10066354",
"snomed_id": "c/276803003"
}
}
},
"therapy": {
"name": "Chemotherapy",
"drugbank_id": "DBCOND0020513",
"meddra_id": "llt/10050693",
"snomed_id": "d/501450016",
"icd10_id": "c/Z51.11"
},
"patient_characteristics": [
{
"name": "No prior therapy for metastatic disease",
"drugbank_id": "DBCOND0130369"
}
],
"genetic_factors": {
"includes": [
{
"name": "HER2",
"synonyms": [
"HER-2",
"HER2/neu",
"..."
],
"gene": {
"name": "ERBB2",
"drugbank_id": "DBCOND0024917",
"uniprot_id": "ac/P04626"
},
"positive_finding": {
"name": "HER2 Positive",
"drugbank_id": "DBCOND0040154",
"meddra_id": "llt/10089726"
},
"negative_finding": {
"name": "HER2 negative",
"drugbank_id": "DBCOND0024919",
"meddra_id": "llt/10077484"
}
}
],
"excludes": []
}
}
}
},
"..."
]
}
This endpoint retrieves a list of product concepts which have indications similar to the indications of the provided product concept.
The results are returned as a JSON object with a “similar_indications” property containing an array of objects representing similar product concepts. Each object in this array has three properties: product_concept (the similar product_concept), from, and to.
From and to describe the relationship between the input product concept and the similar product concept. From and to both contain the same two properties:
- concept, a condition object, and
- indication, an indication object.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/similar_indications
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept for which to retrieve product concepts with similar indications |
View Product Concept Adverse Effects
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0018378/adverse_effects'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Tolcapone",
"drugbank_id": "DB00323"
},
"route": [
"Oral"
],
"dose_form": [],
"evidence_type": [
"clinical_trial"
],
"regions": "US",
"incidences": [
{
"kind": "experimental",
"percent": "42-51%"
},
{
"kind": "placebo",
"percent": "20%"
}
],
"effect": {
"name": "Dyskinesia",
"drugbank_id": "DBCOND0017935",
"meddra_id": "pt/10013916",
"snomed_id": "d/17050018",
"icd10_id": "c/G24.9"
},
"patient_characteristics": [
{
"name": "Parkinson's Disease",
"drugbank_id": "DBCOND0028039",
"meddra_id": "llt/10034008",
"snomed_id": "d/2741287018",
"icd10_id": "c/G20"
}
],
"with_drugs": [
{
"name": "Levodopa",
"drugbank_id": "DB01235"
},
{
"name": "Carbidopa",
"drugbank_id": "DB00190"
}
]
}
]
This returns a list of adverse effects linked to the product concept specified by ID (DrugBank Product Concept ID). This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/adverse_effects
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
include_references |
false | If true, includes the list of references for each adverse effect associated with this product concept. See References for details. |
View Product Concept Contraindications
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0323661/contraindications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Loxapine",
"drugbank_id": "DB00408"
},
"route": [
"Intramuscular"
],
"dose_form": [
"Injection"
],
"regions": "US",
"patient_conditions": [
{
"name": "Comatose",
"drugbank_id": "DBCOND0035758",
"meddra_id": "llt/10058472",
"snomed_id": "d/495581010",
"icd10_id": "c/R40.2"
}
]
}
]
This returns a list of contraindications linked to the product concept specified by ID (DrugBank Product Concept ID). This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/contraindications
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
View Product Concept Boxed Warnings
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0323661/boxed_warnings'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Loxapine",
"drugbank_id": "DB00408"
},
"kind": "warning",
"lab_values": [],
"route": [],
"age_groups": [
"seniors"
],
"risk": {
"name": "Increased mortality",
"drugbank_id": "DBCOND0100456"
},
"patient_characteristics": [
{
"name": "Dementia-related Psychosis",
"drugbank_id": "DBCOND0092686"
}
]
}
]
This returns a list of boxed warnings (Black Box warnings) linked to the product concept specified by ID (DrugBank Product Concept ID). This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/boxed_warnings
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
View Product Concept Parents
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0001930/parents'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Pantoprazole 20 mg Oral",
"display_name": null,
"drugbank_pcid": "DBPC0001929",
"brand": null,
"level": 3,
"route": "Oral",
"form": null,
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1999-01-29",
"regions": {
"us": true,
"canada": true,
"eu": true,
"germany": true,
"colombia": true,
"turkey": true,
"italy": true,
"malaysia": true,
"austria": true,
"indonesia": false,
"thailand": true,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
}
]
This returns a list of product concepts which are more general versions of the product concept specified by <ID>. Each of the returned product concepts is one level more general.
The returned parents can be further refined by passing in a full-text search parameter q. This query parameter works similarity to the medication name search and searches brand names, ingredient names and synonyms as well as ingredient names and synonyms. It also allows the query_type and hit_details parameters to be used.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/parents
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | The text-search query string (optional). | |
| query_type | simple | If set to advanced, allows basic boolean operations in the query parameter q. If set to exact, only returns product concepts containing an exact match to the query string q. Only applies if q is also given. |
| hit_details | false | If set to true, returns additional information on matched brands/ingredients/products for the concept. Only applies if q is also given. |
| withdrawn | false | If set to include, includes product concepts for which all products have been withdrawn. If set to true, includes only product concepts for which all products have been withdrawn. If set to false, includes only product concepts with at least one non-withdrawn product. |
| include_simple_desc | false | If set to true, include simple descriptions for the product concept ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product concept ingredients. |
View Product Concept Children
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0001930/children'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Pantoprazole sodium 20 mg Oral Tablet, coated",
"display_name": null,
"drugbank_pcid": "DBPC0656321",
"brand": null,
"level": 5,
"route": "Oral",
"form": "Tablet, coated",
"strengths": "20 mg",
"standing": "active",
"standing_updated_at": null,
"standing_active_since": "1999-10-07",
"regions": {
"us": false,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": true,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": true,
"mexico": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Pantoprazole as Pantoprazole sodium",
"drug": {
"name": "Pantoprazole",
"drugbank_id": "DB00213"
},
"exact_ingredient": {
"name": "Pantoprazole sodium",
"drugbank_id": "DBSALT000386"
},
"strength": {
"amount": "20.0",
"per": "1.0",
"units": "mg"
}
}
]
}
]
This returns a list of product concepts which are more specific versions of the product concept specified by <ID>. Each of the returned product concepts is one level more specific.
The returned children can be further refined by passing in a full-text search parameter q. This query parameter works similarity to the medication name search and searches brand names, ingredient names and synonyms as well as ingredient names and synonyms. It also allows the query_type and hit_details parameters to be used.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/children
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | The text-search query string (optional). | |
| query_type | simple | If set to advanced, allows basic boolean operations in the query parameter q. If set to exact, only returns product concepts containing an exact match to the query string q. Only applies if q is also given. |
| hit_details | false | If set to true, returns additional information on matched brands/ingredients/products for the concept. Only applies if q is also given. |
| withdrawn | false | If set to include, includes product concepts for which all products have been withdrawn. If set to true, includes only product concepts for which all products have been withdrawn. If set to false, includes only product concepts with at least one non-withdrawn product. |
| include_simple_desc | false | If set to true, include simple descriptions for the product concept ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product concept ingredients. |
View Product Concepts by RxNorm Concept ID (RXCUI)
curl -L 'https://api.drugbank.com/v1/product_concepts/rxnorm/6387'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Lidocaine",
"display_name": null,
"drugbank_pcid": "DBPC0005263",
"brand": null,
"level": 1,
"route": null,
"form": null,
"strengths": null,
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1948-11-19",
"regions": {
"us": true,
"canada": true,
"eu": false,
"germany": false,
"colombia": true,
"turkey": true,
"italy": true,
"malaysia": true,
"austria": true,
"indonesia": true,
"thailand": true,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [
{
"name": "lidocaine",
"RXCUI": "6387"
}
],
"ingredients": [
{
"name": "Lidocaine",
"drug": {
"name": "Lidocaine",
"drugbank_id": "DB00281"
}
}
]
}
]
This returns a list of product concepts which have been mapped to the provided RXCUI. The relationship between RXCUI and DrugBank product concepts is many-to-many. If the specified RXCUI is not understood, or has not yet been imported into DrugBank, then a 404 error will be raised. If the RXCUI is understood, but the specified RxNorm concept is not considered equivalent to any DrugBank product concept, then an empty array will be returned. This API supports pagination, although results will rarely, if ever be paginated with the default per-page setting of 50 results per page.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/rxnorm/<RXCUI>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The RXCUI of the RxNorm concept for which to retrieve product concepts. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| withdrawn | false | If set to include, includes product concepts for which all products have been withdrawn. If set to true, includes only product concepts for which all products have been withdrawn. If set to false, includes only product concepts with at least one non-withdrawn product. |
| include_simple_desc | false | If set to true, include simple descriptions for the product concept ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product concept ingredients. |
View Product Concept Revocation
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0151796/revocation'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"drugbank_pcid": "DBPC0151796",
"alternate_drugbank_pcids": [],
"standing": "revoked",
"revoked_at": "2018-01-11",
"historical_data": {
"name": "Estradiol valerate / Testosterone Enanthate Intramuscular",
"route": "Intramuscular",
"rxnorm_ids": [],
"ingredients": [
{
"drug": {
"name": "Testosterone",
"drugbank_id": "DB00624"
},
"exact_ingredient": {
"name": "Testosterone Enanthate",
"drugbank_id": "DBSALT001030"
}
},
{
"drug": {
"name": "Estradiol",
"drugbank_id": "DB00783"
},
"exact_ingredient": {
"name": "Estradiol valerate",
"drugbank_id": "DBSALT000068"
}
}
]
},
"suggested_replacements": [
{
"name": "Estradiol valerate 6.5 mg / Testosterone enanthate 100 mg /mL Intramuscular",
"drugbank_pcid": "DBPC0734281",
"regions": {
"us": false,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": false,
"mexico": false
}
},
{
"name": "Estradiol valerate 6.5 mg / Testosterone enanthate 100 mg /mL Intramuscular Solution",
"drugbank_pcid": "DBPC0734425",
"regions": {
"us": false,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": false,
"mexico": false
}
},
"..."
]
}
When a drug or product ingredient is revoked, the related product concepts must also be revoked. They refer to DrugBank entities which no longer exist, so they cannot be properly represented. However, a historical snapshot of its data is captured when a product concept is revoked. This data can be accessed through the revocation endpoint, which will also attempt to suggest product concepts which may be suitable replacements for the revoked concept.
Plese note that some or all of the referenced ingredients may no longer be available.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/revocation
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept for which to retrieve the revocation data. |
Search duplicate therapies
curl -L 'https://api.drugbank.com/v1/product_concepts/duplicate_therapies?product_concept_id=DBPC0251172,DBPC0011839'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"duplicate_ingredients": [
{
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
},
"product_concepts": [
{
"name": "Acetaminophen 500 mg Oral Tablet, film coated [Panadol]",
"display_name": "Panadol 500 mg Oral Tablet, film coated",
"drugbank_pcid": "DBPC0251172",
"brand": "Panadol",
"level": 5,
"route": "Oral",
"form": "Tablet, film coated",
"strengths": "500 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2011-03-18",
"regions": {
"us": true,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": false,
"mexico": false
},
"rxnorm_concepts": [
{
"name": "acetaminophen 500 MG Oral Tablet [Panadol]",
"RXCUI": "200977"
}
],
"ingredients": [
{
"name": "Acetaminophen",
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
},
"strength": {
"amount": "500.0",
"per": "1.0",
"units": "mg"
}
}
]
},
{
"name": "Acetaminophen 160 mg /5mL Oral Suspension",
"display_name": null,
"drugbank_pcid": "DBPC0011839",
"brand": null,
"level": 4,
"route": "Oral",
"form": "Suspension",
"strengths": "160 mg /5mL",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1989-08-15",
"regions": {
"us": true,
"canada": true,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": true,
"singapore": false,
"mexico": false
},
"rxnorm_concepts": [
{
"name": "acetaminophen 33.3 MG/ML Oral Solution",
"RXCUI": "307684"
},
{
"name": "acetaminophen 100 MG/ML Oral Solution",
"RXCUI": "238159"
},
"..."
],
"ingredients": [
{
"name": "Acetaminophen",
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
},
"strength": {
"amount": "160.0",
"per": "5.0",
"units": "mg/mL"
}
}
]
}
]
}
],
"duplicate_therapies": [
{
"condition": {
"title": "Severe Pain",
"drugbank_id": "DBCOND0066540"
},
"kind": "treatment_of",
"duplicates": [
{
"product_concept": {
"name": "Acetaminophen 160 mg /5mL Oral Suspension",
"display_name": null,
"drugbank_pcid": "DBPC0011839",
"brand": null,
"level": 4,
"route": "Oral",
"form": "Suspension",
"strengths": "160 mg /5mL",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1989-08-15",
"regions": {
"us": true,
"canada": true,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": true,
"singapore": false,
"mexico": false
},
"rxnorm_concepts": [
{
"name": "acetaminophen 33.3 MG/ML Oral Solution",
"RXCUI": "307684"
},
{
"name": "acetaminophen 100 MG/ML Oral Solution",
"RXCUI": "238159"
},
"..."
],
"ingredients": [
{
"name": "Acetaminophen",
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
},
"strength": {
"amount": "160.0",
"per": "5.0",
"units": "mg/mL"
}
}
]
},
"indication": {
"kind": "adjunct_therapy_in_treatment_of",
"off_label": false,
"otc_use": false,
"route": [],
"dose_form": [],
"dose_strength": [],
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
},
"regions": "US",
"condition": {
"name": "Severe Pain",
"drugbank_id": "DBCOND0066540",
"snomed_id": "d/127745015",
"icd10_id": "c/R52",
"modification_of": {
"base": {
"name": "Pain",
"drugbank_id": "DBCOND0012160",
"meddra_id": "llt/10033371",
"snomed_id": "d/253262010",
"icd10_id": "c/R52"
},
"severity": {
"includes": [
"severe"
],
"excludes": []
}
}
},
"with_therapies": [
{
"name": "Opioids",
"drugbank_id": "DBCOND0037703",
"meddra_id": "llt/10063231",
"snomed_id": "d/107481013"
}
]
}
},
{
"product_concept": {
"name": "Acetaminophen 500 mg Oral Tablet, film coated [Panadol]",
"display_name": "Panadol 500 mg Oral Tablet, film coated",
"drugbank_pcid": "DBPC0251172",
"brand": "Panadol",
"level": 5,
"route": "Oral",
"form": "Tablet, film coated",
"strengths": "500 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2011-03-18",
"regions": {
"us": true,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": false,
"mexico": false
},
"rxnorm_concepts": [
{
"name": "acetaminophen 500 MG Oral Tablet [Panadol]",
"RXCUI": "200977"
}
],
"ingredients": [
{
"name": "Acetaminophen",
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
},
"strength": {
"amount": "500.0",
"per": "1.0",
"units": "mg"
}
}
]
},
"indication": {
"kind": "adjunct_therapy_in_treatment_of",
"off_label": false,
"otc_use": false,
"route": [],
"dose_form": [],
"dose_strength": [],
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
},
"regions": "US",
"condition": {
"name": "Severe Pain",
"drugbank_id": "DBCOND0066540",
"snomed_id": "d/127745015",
"icd10_id": "c/R52",
"modification_of": {
"base": {
"name": "Pain",
"drugbank_id": "DBCOND0012160",
"meddra_id": "llt/10033371",
"snomed_id": "d/253262010",
"icd10_id": "c/R52"
},
"severity": {
"includes": [
"severe"
],
"excludes": []
}
}
},
"with_therapies": [
{
"name": "Opioids",
"drugbank_id": "DBCOND0037703",
"meddra_id": "llt/10063231",
"snomed_id": "d/107481013"
}
]
}
}
]
}
]
}
Check whether product concepts share the same ingredients or treat the same conditions.
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| product_concept_id | A comma-separated list of DrugBank product concept IDs |
This returns two high level sections: duplicate_ingredients and duplicate_therapies.
duplicate_ingredients will return the entered product concepts grouped by their shared ingredients. Each entry of the array will contain the common drug and the product_concepts containing that drug.
duplicate_therapies will return the entered product concepts, along with their indications, grouped by the closest common condition that they treat. Each entry of the array will contain the common condition and kind and an array of duplicates. Entries in the duplicates array will be one of the entered product concepts along with the indication related to this condition.
Get the list of current FDA labels available for a product concept
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0426158/labels'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"id": "08d4f62f-fa28-6afc-d4bf-311386d4ad35",
"set_id": "0d3a966f-f937-05a8-a90f-5aa52ebbd613",
"version": 14,
"current": true,
"title": "Zestoretic - lisinopril and hydrochlorothiazide TABLET",
"product_type": "HUMAN PRESCRIPTION DRUG LABEL",
"effective_on": "2021-07-01",
"labeler_name": "Almatica Pharma Inc.",
"first_product_marketing_started_on": "2015-04-15",
"last_product_marketing_ended_on": null,
"ndc_product_codes": [
"52427-0437"
],
"pmg_section_count": 1,
"hpi_section_count": 17,
"sli_section_count": 5,
"all_section_count": 21
}
]
This endpoint retrieves the list of current FDA labels for a product concept.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/labels
View Drug Alternatives for a Product Concept
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC503562/therapeutic_alternatives'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Amoxicillin",
"drugbank_id": "DB01060",
"categories": [
{
"drugbank_id": "DBCAT003682",
"name": "Aminopenicillins",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"categorization_kinds": [
"therapeutic"
],
"synonyms": [],
"description": null,
"drugs": [
{
"name": "Ampicillin",
"drugbank_id": "DB00415"
}
]
},
{
"drugbank_id": "DBCAT000702",
"name": "Penicillins",
"mesh_id": "D010406",
"mesh_tree_numbers": [
"D02.065.589.099.750",
"D02.886.108.750",
"..."
],
"atc_code": "S01AA19",
"atc_level": 5,
"categorization_kinds": [
"therapeutic",
"indexing"
],
"synonyms": [
"Amcill",
"Aminobenzyl Penicillin",
"..."
],
"description": "A group of antibiotics that contain 6-aminopenicillanic acid with a side chain attached to the 6-amino group...",
"drugs": [
{
"name": "Cloxacillin",
"drugbank_id": "DB01147"
},
{
"name": "Pivmecillinam",
"drugbank_id": "DB01605"
},
"..."
]
}
]
}
]
This endpoint retrieves lists of drugs as therapeutic alternatives based on the therapeutic categorizations of the ingredients associated with the provided product concept.
Related product concepts to the drug alternatives can then be found using this endpoint.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/therapeutic_alternatives
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve the linked drug alternatives. |
Drugs
Get a specific drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00316'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"drugbank_id": "DB00316",
"name": "Acetaminophen",
"cas_number": "103-90-2",
"annotation_status": "complete",
"availability_by_region": [
{
"region": "ca",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
{
"region": "us",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
"..."
],
"description": "Acetaminophen (paracetamol), also commonly known as _Tylenol_, is the most commonly taken analgesic worldwide and is recommended as first-line therapy ...",
"simple_description": "A medication used to reduce fever and treat pain.",
"clinical_description": "An analgesic drug used alone or in combination with opioids for pain management, and as an antipyretic agent.",
"synonyms": [
"4-(Acetylamino)phenol",
"4-acetamidophenol",
"..."
],
"pharmacology": {
"indication_description": "In general, acetaminophen is used for the treatment of mild to moderate pain and reduction of fever...",
"pharmacodynamic_description": "Animal and clinical studies have determined that acetaminophen has both antipyretic and analgesic effects...",
"mechanism_of_action_description": "According to its FDA labeling, acetaminophen's exact mechanism of action has not been fully established[Label] - despite this, it is often categorized ...",
"absorption": "Acetaminophen has 88% oral bioavailability and reaches its highest plasma concentration 90 minutes after ingestion...",
"protein_binding": "The binding of acetaminophen to plasma proteins is low (ranging from 10% to 25%), when given at therapeutic doses.[Label]",
"volume_of_distribution": [
"Volume of distribution is about 0..."
],
"clearance": [
"Adults: 0.27 L/h/kg following a 15 mg/kg intravenous (IV) dose.[Label]",
"Children: 0.34 L/h/kg following a 15 mg/kg intravenous (IV dose).[Label]"
],
"half_life": "The half-life for adults is 2...",
"route_of_elimination": "Acetaminophen metabolites are mainly excreted in the urine...",
"toxicity_description": "LD50 = 338 mg/kg (oral, mouse); LD50 = 1944 mg/kg (oral, rat)[F4133]\r\n\r\n**Overdose and liver toxicity** \r\n\r\nAcetaminophen overdose may be manifested by..."
},
"food_interactions": [
"Avoid alcohol. Alcohol may increase the risk of hepatotoxicity.",
"Take with or without food. The absorption is unaffected by food."
],
"identifiers": {
"drugbank_id": "DB00316",
"inchi": "InChI=1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10)",
"inchikey": "RZVAJINKPMORJF-UHFFFAOYSA-N",
"atc_codes": [
{
"code": "N02BE71",
"title": "paracetamol, combinations with psycholeptics",
"combination": true
},
{
"code": "N02BE01",
"title": "paracetamol",
"combination": false
},
"..."
]
},
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000041",
"name": "Analgesics",
"mesh_id": "D000700",
"mesh_tree_numbers": [
"D27.505.696.663.850.014",
"D27.505.954.427.040"
],
"atc_code": "N02",
"atc_level": 2,
"synonyms": [
"Agents, Analgesic",
"Analgesic Agents",
"..."
],
"description": "Compounds that show activity in animal models of human PAIN such as tail flick and hot plate assays."
}
]
}
This endpoint retrieves a specific drug based on DrugBank ID.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
include_references |
false | If true, includes the list of references for this drug. See References for details. |
Get products linked with a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00316/products'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"ndc_product_code": "52124-0111",
"originator_ndc_product_code": "52124-0111",
"dpd_id": null,
"ema_product_code": null,
"ema_ma_number": null,
"pzn": null,
"co_product_id": null,
"tr_product_id": null,
"it_product_id": null,
"my_product_id": null,
"at_product_id": null,
"idn_product_id": null,
"th_product_id": null,
"sg_product_id": null,
"mx_registration_id": null,
"mx_product_id": null,
"name": "10 Person ANSI",
"prescribable_name": "Acetaminophen / Acetylsalicylic acid / Bacitracin zinc / Benzalkonium chloride / Benzocaine / Ethanol / Ibuprofen / Lidocaine / Neomycin sulfate / Poly...",
"started_marketing_on": "2010-04-24",
"ended_marketing_on": null,
"approved_on": null,
"schedule": null,
"dosage_form": "Kit",
"route": "Ophthalmic; Oral; Topical",
"application_number": "part333",
"generic": false,
"otc": true,
"approved": false,
"country": "US",
"mixture": true,
"allergenic": false,
"cosmetic": false,
"vaccine": false,
"ingredients": [
{
"name": "Benzalkonium",
"drugbank_id": "DB11105",
"strength": {
"number": "0.13",
"unit": "g/100g"
}
},
{
"name": "Lidocaine",
"drugbank_id": "DB00281",
"strength": {
"number": "0.5",
"unit": "1/100g"
}
},
"..."
],
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000853",
"name": "Aminoglycoside Antibacterials",
"mesh_id": "D000617",
"mesh_tree_numbers": [
"D09.408.051"
],
"atc_code": "J01G",
"atc_level": 3,
"synonyms": [
"Amino-glycoside Antibacterials",
"Amino-glycoside Antibiotics",
"..."
],
"description": "A category of amino glycoside antibacterials..."
},
{
"drugbank_id": "DBCAT000041",
"name": "Analgesics",
"mesh_id": "D000700",
"mesh_tree_numbers": [
"D27.505.696.663.850.014",
"D27.505.954.427.040"
],
"atc_code": "N02",
"atc_level": 2,
"synonyms": [
"Agents, Analgesic",
"Analgesic Agents",
"..."
],
"description": "Compounds that show activity in animal models of human PAIN such as tail flick and hot plate assays."
},
"..."
],
"labeller": {
"name": "Genuine First Aid"
},
"images": []
}
]
This endpoint retrieves a list of products linked to a drug, based on DrugBank ID. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/products
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked products. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| include_simple_desc | false | If set to true, include simple descriptions for the product ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product ingredients. |
Filters
Products can be narrowed down by appending a filter to the url in the following format:
https://api.drugbank.com/v1/drugs/<ID>/products/<filter>
For example:
https://api.drugbank.com/v1/drugs/<ID>/products/generics
| Filter | Description |
|---|---|
| generics | Returns only generic i.e. unbranded products. |
Get product concepts linked with a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00316/product_concepts'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Acetaminophen / Levomenthol Oral Solution",
"display_name": null,
"drugbank_pcid": "DBPC0869320",
"brand": null,
"level": 3,
"route": "Oral",
"form": "Solution",
"strengths": null,
"standing": "active",
"standing_updated_at": null,
"standing_active_since": null,
"regions": {
"us": false,
"canada": true,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": false,
"mexico": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Acetaminophen",
"drug": {
"name": "Acetaminophen",
"drugbank_id": "DB00316"
}
},
{
"name": "Levomenthol",
"drug": {
"name": "Levomenthol",
"drugbank_id": "DB00825"
}
}
]
}
]
This endpoint retrieves a list of product concepts linked to a drug, based on DrugBank ID. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/product_concepts
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked product concepts. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| level | The product concept level to filter by (optional). | |
| min_level | The minimum product concept level to return (optional). | |
| min_level | The maximum product concept level to return (optional). | |
| unbranded_only | false | If true, returns only product concepts without an associated brand. |
Get categories for a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00993/categories'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DBCAT000276",
"name": "Antimetabolites",
"mesh_id": "D000963",
"mesh_tree_numbers": [
"D27.505.519.186",
"D27.888.569.042"
],
"atc_code": "L01B",
"atc_level": 3,
"categorization_kind": "therapeutic",
"synonyms": [],
"description": "Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways..."
}
]
Returns an array of categories for a drug, based on the DrugBank ID.
The category type atc or mesh can be appended on to the end of the url to specify which hierarchy to retrieve the categories from. If left blank, DrugBank categories will be returned.
This endpoint supports pagination.
See Common Category Query Parameter Values for query parameters that affect the results of this request.
Shows all categories for the drug regardless of the value of source, but parent/child relationships will be limited based on source.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/categories/<hierarchy (optional)>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked categories. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| categorization_kind | The categorization kind to filter by (optional). |
Get indications linked with a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00675/indications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"kind": "used_in_combination_to_treat",
"off_label": true,
"otc_use": false,
"route": [
"Oral"
],
"dose_form": [],
"dose_strength": [],
"combination_type": "regimen",
"drug": {
"name": "Tamoxifen",
"drugbank_id": "DB00675"
},
"condition": {
"name": "Desmoid tumour",
"drugbank_id": "DBCOND0016232",
"meddra_id": "llt/10059352",
"snomed_id": "d/3009352010",
"icd10_id": "c/C49.9",
"modification_of": {
"base": {
"name": "Fibromatosis",
"drugbank_id": "DBCOND0016239",
"meddra_id": "llt/10049444",
"snomed_id": "d/33542010",
"icd10_id": "c/M72.9"
},
"severity": {
"includes": [
"aggressive"
],
"excludes": []
}
}
},
"combination_drugs": [
{
"name": "Sulindac",
"drugbank_id": "DB00605"
}
]
}
]
This endpoint retrieves a list of indications linked to a drug, based on DrugBank ID. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/indications
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked indications. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| off_label | null | Limits results by the value of the off_label attribute of the indications. |
| otc_use | null | Limits results by the value of the otc_use attribute of the indications. |
| kind | null | Limits results by the value of the kind attribute of the indications. |
View drugs with similar indications
curl -L 'https://api.drugbank.com/v1/drugs/DB00675/similar_indications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"similar_indications": [
{
"drug": {
"name": "Anastrozole",
"drugbank_id": "DB01217"
},
"from": {
"concept": {
"title": "Breast Cancer",
"drugbank_id": "DBCOND0028036"
},
"indication": {
"kind": "adjunct_therapy_in_prevention_of",
"off_label": false,
"otc_use": false,
"route": [
"Oral"
],
"dose_form": [
"Liquid",
"Solution",
"..."
],
"dose_strength": [],
"age_groups": [
"adult"
],
"drug": {
"name": "Tamoxifen",
"drugbank_id": "DB00675"
},
"regions": "US",
"condition": {
"name": "Contralateral Breast Cancer",
"drugbank_id": "DBCOND0111583",
"meddra_id": "llt/10054784",
"snomed_id": "c/254837009",
"icd10_id": "c/C50"
},
"condition_associated_with": [
{
"name": "Breast Cancer",
"drugbank_id": "DBCOND0028036",
"meddra_id": "llt/10006187",
"snomed_id": "d/379662011",
"icd10_id": "c/C50.9",
"modification_of": {
"location": "Breast",
"base": {
"name": "Cancer",
"drugbank_id": "DBCOND0020966",
"meddra_id": "llt/10007050",
"snomed_id": "d/142665016",
"icd10_id": "c/C80"
}
}
}
]
}
},
"to": {
"concept": {
"title": "Advanced Breast Cancer",
"drugbank_id": "DBCOND0031019"
},
"indication": {
"kind": "treatment_of",
"off_label": false,
"otc_use": false,
"sex_group": "female",
"route": [
"Oral"
],
"dose_form": [],
"dose_strength": [],
"age_groups": [
"postmenopausal"
],
"drug": {
"name": "Anastrozole",
"drugbank_id": "DB01217"
},
"regions": "US",
"condition": {
"name": "Advanced Breast Cancer",
"drugbank_id": "DBCOND0031019",
"meddra_id": "llt/10072737",
"snomed_id": "c/254837009",
"icd10_id": "c/C50",
"modification_of": {
"condition_stage": "advanced",
"base": {
"name": "Breast Cancer",
"drugbank_id": "DBCOND0028036",
"meddra_id": "llt/10006187",
"snomed_id": "d/379662011",
"icd10_id": "c/C50.9"
}
}
},
"patient_characteristics": [
{
"name": "Disease progression with tamoxifen therapy",
"drugbank_id": "DBCOND0100394"
}
]
}
}
},
{
"drug": {
"name": "Letrozole",
"drugbank_id": "DB01006"
},
"from": {
"concept": {
"title": "Breast Cancer",
"drugbank_id": "DBCOND0028036"
},
"indication": {
"kind": "adjunct_therapy_in_prevention_of",
"off_label": false,
"otc_use": false,
"route": [
"Oral"
],
"dose_form": [
"Liquid",
"Solution",
"..."
],
"dose_strength": [],
"age_groups": [
"adult"
],
"drug": {
"name": "Tamoxifen",
"drugbank_id": "DB00675"
},
"regions": "US",
"condition": {
"name": "Contralateral Breast Cancer",
"drugbank_id": "DBCOND0111583",
"meddra_id": "llt/10054784",
"snomed_id": "c/254837009",
"icd10_id": "c/C50"
},
"condition_associated_with": [
{
"name": "Breast Cancer",
"drugbank_id": "DBCOND0028036",
"meddra_id": "llt/10006187",
"snomed_id": "d/379662011",
"icd10_id": "c/C50.9",
"modification_of": {
"location": "Breast",
"base": {
"name": "Cancer",
"drugbank_id": "DBCOND0020966",
"meddra_id": "llt/10007050",
"snomed_id": "d/142665016",
"icd10_id": "c/C80"
}
}
}
]
}
},
"to": {
"concept": {
"title": "Advanced Breast Cancer",
"drugbank_id": "DBCOND0031019"
},
"indication": {
"kind": "treatment_of",
"off_label": false,
"otc_use": false,
"sex_group": "female",
"route": [
"Oral"
],
"dose_form": [
"Tablet"
],
"dose_strength": [
"2.5mg"
],
"drug": {
"name": "Letrozole",
"drugbank_id": "DB01006"
},
"regions": "US",
"condition": {
"name": "Advanced Breast Cancer",
"drugbank_id": "DBCOND0031019",
"meddra_id": "llt/10072737",
"snomed_id": "c/254837009",
"icd10_id": "c/C50",
"modification_of": {
"condition_stage": "advanced",
"base": {
"name": "Breast Cancer",
"drugbank_id": "DBCOND0028036",
"meddra_id": "llt/10006187",
"snomed_id": "d/379662011",
"icd10_id": "c/C50.9"
}
}
},
"patient_characteristics": [
{
"name": "Post-Menopausal",
"drugbank_id": "DBCOND0034437",
"meddra_id": "llt/10051775",
"snomed_id": "d/3005258016"
}
]
}
}
},
"..."
]
}
This endpoint retrieves a list of drugs which have indications similar to the indications of the provided drug.
The results are returned as a JSON object with a “similar_indications” property containing an array of objects representing similar drugs. Each object in this array has three properties: drug (the similar drug), from, and to.
From and to describe the relationship between the input drug and the similar drug. From and to both contain the same two properties:
- concept, a condition object, and
- indication, an indication object.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/similar_indications
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug for which to retrieve drugs with similar indications |
Get drug-drug interactions for a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00675/ddi?severity=moderate'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"ingredient": {
"drugbank_id": "DB00675",
"name": "Tamoxifen"
},
"affected_ingredient": {
"drugbank_id": "DB08496",
"name": "(R)-warfarin"
},
"description": "The risk or severity of bleeding can be increased when Tamoxifen is combined with (R)-warfarin.",
"extended_description": "Coadministration of tamoxifen with anticoagulant drugs has been shown to produce an increase in the anticoagulant effect producing cases of bleeding...",
"action": "increase_specific_adverse_effects",
"severity": "moderate",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_1",
"management": "Concomitant use of tamoxifen with vitamin K antagonists should be avoided..."
}
]
This endpoint retrieves a list of drug-drug interactions linked to a drug, based on DrugBank ID. The ingredient refers to the specified drug, while the affected_ingredient is the drug it interacts with. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/ddi
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked interactions. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| severity | null | Limits results by the severity of the interactions. May be major, moderate, or minor. |
| evidence_level | null | Limits results by the evidence level of the interactions. May be level_1 or level_2. |
| available_only | false | If true, only includes interactions with currently available drugs. If a region is specified, the drugs must also be available in that region. |
| include_references | false | If true, includes the references for each interaction. See References for details on the format. |
Get adverse effects linked with a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00316/adverse_effects'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"route": [
"Intravenous"
],
"dose_form": [],
"evidence_type": [
"clinical_trial"
],
"admin": "multiple dose",
"regions": "US",
"age_groups": [
"adult"
],
"incidences": [
{
"kind": "experimental",
"percent": "34%"
},
{
"kind": "placebo",
"percent": "31%"
}
],
"effect": {
"name": "Nausea",
"drugbank_id": "DBCOND0010699",
"meddra_id": "pt/10028813",
"snomed_id": "d/122688013",
"icd10_id": "c/R11.0"
}
}
]
This endpoint retrieves a list of adverse effects linked to a drug, based on DrugBank ID. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/adverse_effects
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked adverse effects. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
include_references |
false | If true, includes the list of references for each adverse effect associated with this drug. See References for details. |
Get contraindications linked with a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00316/contraindications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"route": [],
"dose_form": [],
"hypersensitivity": [
"true"
],
"lab_values": [],
"recommended_actions": [],
"regions": "US"
},
{
"route": [],
"dose_form": [],
"hypersensitivity": [],
"lab_values": [],
"recommended_actions": [],
"regions": "US",
"patient_conditions": [
{
"name": "Severe, active Liver Disease",
"drugbank_id": "DBCOND0112340",
"modification_of": {
"condition_status": "active",
"base": {
"name": "Liver Disease",
"drugbank_id": "DBCOND0028338",
"meddra_id": "llt/10045808",
"snomed_id": "d/104462011",
"icd10_id": "c/K76.9"
},
"severity": {
"includes": [
"severe"
],
"excludes": []
}
}
}
]
},
"..."
]
This endpoint retrieves a list of contraindications linked to a drug, based on DrugBank ID. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/contraindications
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked contraindications. |
Get boxed warnings linked with a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00316/boxed_warnings'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"kind": "warning",
"lab_values": [],
"route": [],
"dose_form": [],
"risk": {
"name": "Hepatotoxicity",
"drugbank_id": "DBCOND0013066",
"meddra_id": "llt/10019851",
"snomed_id": "d/80589010",
"icd10_id": "c/K71.9"
}
},
{
"kind": "warning",
"recommendation": "Do not exceed recommended daily maximum limits",
"lab_values": [],
"route": [],
"dose_form": [],
"risk": {
"name": "Liver Failure",
"drugbank_id": "DBCOND0031848",
"meddra_id": "llt/10019663",
"snomed_id": "d/303485018",
"icd10_id": "c/K72.9"
}
},
"..."
]
This endpoint retrieves a list of boxed warnings (Black Box warnings) linked to a drug, based on DrugBank ID. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/boxed_warnings
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked boxed warnings. |
Get packages for a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00316/packages'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"package_ndc_code": "16590-0023-90",
"originator_package_ndc_code": "16590-023-90",
"description": "90 TABLET IN 1 BOTTLE",
"full_description": "90 TABLET IN 1 BOTTLE",
"amount": "90",
"unit": "1",
"form": "BOTTLE",
"product": {
"ndc_product_code": "16590-0023",
"name": "ACETAMINOPHEN AND CODEINe"
}
},
{
"package_ndc_code": "21695-0242-28",
"originator_package_ndc_code": "21695-242-28",
"description": "28 TABLET IN 1 BOTTLE",
"full_description": "28 TABLET IN 1 BOTTLE",
"amount": "28",
"unit": "1",
"form": "BOTTLE",
"product": {
"ndc_product_code": "21695-0242",
"name": "Acetaminophen and Codeine Phosphate"
}
},
"..."
]
This endpoint retrieves a list of top level packages for a drug, based on DrugBank ID. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/packages
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the packages. |
Get the list of current FDA labels available for a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB00722/labels'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"id": "198bd070-05db-bad4-e063-6394a90a4830",
"set_id": "fab269ea-9513-3702-e053-6394a90ada0d",
"version": 2,
"current": true,
"title": "Lisinopril - Lisinopril TABLET",
"product_type": "HUMAN PRESCRIPTION DRUG LABEL",
"effective_on": "2024-05-28",
"labeler_name": "Pharmasource Meds, LLC",
"first_product_marketing_started_on": "2023-05-02",
"last_product_marketing_ended_on": "2025-02-28",
"ndc_product_codes": [
"82982-0066"
],
"pmg_section_count": 1,
"hpi_section_count": 18,
"sli_section_count": 9,
"all_section_count": 27
}
]
This endpoint retrieves the list of current FDA labels for a drug, linked through drug products.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/labels
View Therapeutic Alternatives for a Drug
curl -L 'https://api.drugbank.com/v1/drugs/DB09156/therapeutic_alternatives'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DBCAT003315",
"name": "Radiographic Contrast Agent",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"categorization_kind": "therapeutic",
"synonyms": [
"Radiographic Contrast Agents",
"Radiographic Contrast Drug",
"..."
],
"description": null,
"drugs": [
{
"name": "Ioxilan",
"drugbank_id": "DB09135"
},
{
"name": "Diatrizoate",
"drugbank_id": "DB00271"
},
"..."
]
}
]
This endpoint retrieves lists of drugs as therapeutic alternatives based on the provided drug’s therapeutic categorizations.
The results are returned as individual categories where the provided drug is considered therapeutic for that category, each with a separate list of drug alternatives.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/therapeutic_alternatives
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the linked therapeutic alternatives. |
Drug-Drug Interactions
Provide a list of DrugBank drug ids to get a list of interactions between the given drugs, and/or a list of products to get a list of interactions between ingredients in the given products. Products can be specified by product concept ID or regional product code.
The number of query terms that can be provided in a single query is limited to 40. These query parameters can be sent as url parameters or as JSON in the body of a POST request. A single request can mix different query terms, for instance, drug ids with product concept ids.
Drug-Drug Interactions (DDI) Plugin
Our DDI Plugin provides a “plug and play” alternative to implementing all of our “Find DDI” endpoints below:
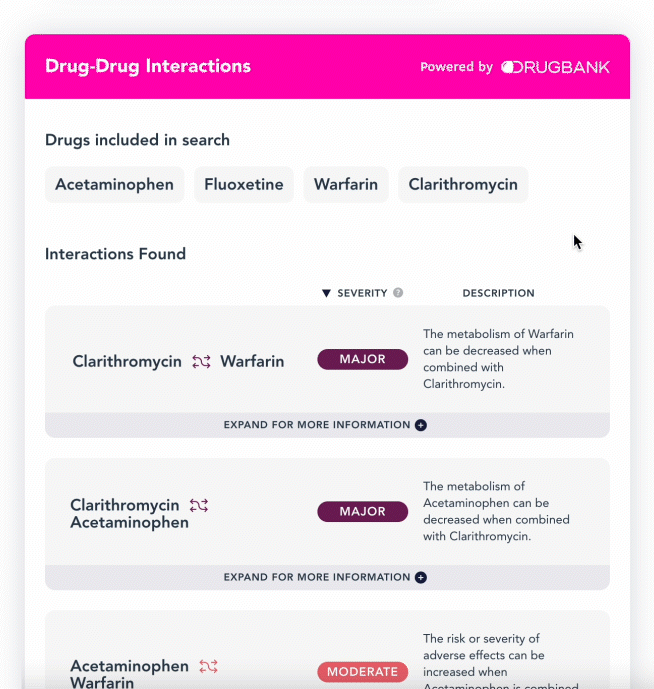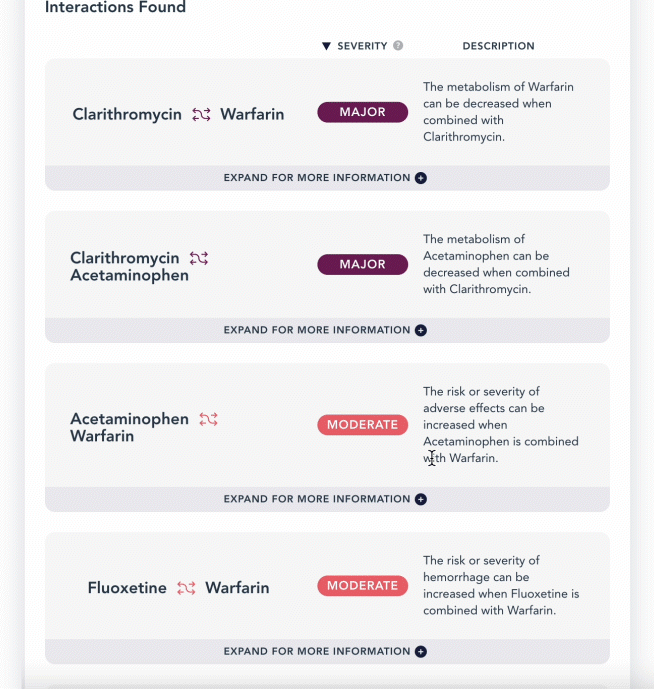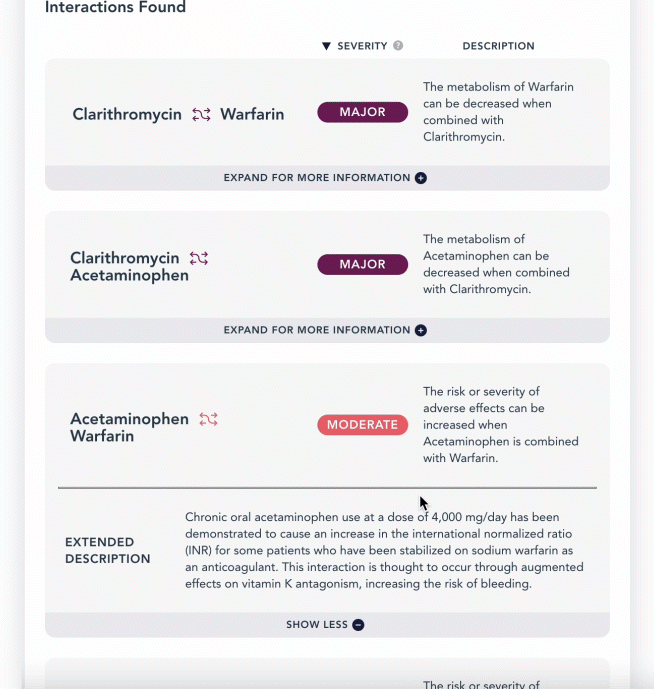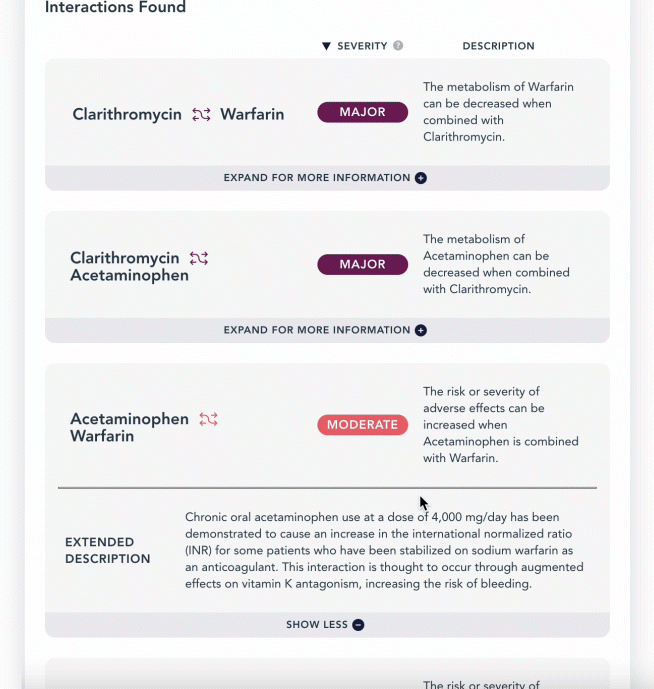
As a standard web component, it is easy to integrate to suit your needs and comes with a number of available properties to fine-tune its functionality. This plugin can be used together with our Medication Search Plugin. For more information, please see this public repository.
Common Query Parameter Values
Return a list of drug interactions, including their references:
curl -L 'https://api.drugbank.com/v1/ddi?drugbank_id=DB01236,DB01363&include_references=true'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"interactions": [
{
"ingredient": {
"drugbank_id": "DB01236",
"name": "Sevoflurane"
},
"affected_ingredient": {
"drugbank_id": "DB01363",
"name": "Ephedra sinica root"
},
"description": "Sevoflurane may increase the arrhythmogenic activities of Ephedra sinica root.",
"extended_description": "The use of ephedra is currently either banned or strongly recommended against as being unsafe in many countries [L3650, L3651, F648]...",
"action": "increase_specific_effects",
"severity": "major",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_1",
"management": "Avoid coadministration of ephedra with cyclopropane or halogenated hydrocarbon anesthetics whenever possible...",
"references": {
"literature_references": [
{
"ref_id": "A35004",
"pubmed_id": 17594156,
"citation": "Hahm TS, Lee JJ, Yang MK, Kim JA: Risk factors for an intraoperative arrhythmia during esophagectomy..."
},
{
"ref_id": "A35071",
"pubmed_id": 18941968,
"citation": "Himmel HM: Mechanisms involved in cardiac sensitization by volatile anesthetics: general applicability to halogenated hydrocarbons? Crit Rev Toxicol..."
},
"..."
],
"textbooks": [],
"external_links": [
{
"ref_id": "L3620",
"title": "Electronic Medicines Compendium: Ephedrine Hydrochloride Injection 3MG PER ML Monograph",
"url": "https://www.medicines.org.uk/emc/product/3593/smpc"
},
{
"ref_id": "L3650",
"title": "National Institutes of Health: FDA Prohibits Sales of Dietary Supplements Containing Ephedra",
"url": "https://ods.od.nih.gov/Health_Information/ephedra.aspx"
},
"..."
],
"attachments": [
{
"ref_id": "F648",
"title": "European Food Safety Authority: Scientific Opinion on Safety Evaluation of Ephedra Species for use in Food",
"url": "//s3-us-west-2.amazonaws.com/drugbank/cite_this/attachments/files/000/000/648/original/j.efsa.2013.3467.pdf?1531845562"
}
]
}
}
],
"total_results": 1
}
For any interaction query the parameters listed in the table below can be used:
| Parameter | Default | Description |
|---|---|---|
| severity | null | Limits results by the severity of the interactions. May be major, moderate, or minor. |
| evidence_level | null | Limits results by the evidence level of the interactions. May be level_1 or level_2. |
| include_references | false | If true, includes the references for each interaction. See References for details on the format. |
An explanation of some of the interaction properties:
| Property | Type | Description |
|---|---|---|
| severity | string | The severity of this drug interaction; either minor, moderate, or major. |
| action | string | The resulting effect of this interaction on the pharmacological activity of the drug. |
| evidence_level | string | level_1: Mentioned in the the drug monograph (FDA, Health Canada, EMA, etc) and has been confirmed in clinical studies (cohort, case-control, case study etc.)level_2: Has been confirmed in at least 1 cohort, case-control, or case study and may or not be mentioned in a drug monograph. |
Find DDI with Local Product ID
Return a list of
moderatedrug interactions involving the products with the NDC codes0169-5174and0013-2626:
curl -L 'https://api.drugbank.com/v1/ddi?ndc=0169-5174,0013-2626&severity=moderate'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"interactions": [
{
"product_ndc_product_codes": [
"00169-5174"
],
"product_name": "Activella",
"product_ingredient": {
"drugbank_id": "DB00783",
"name": "Estradiol"
},
"affected_product_ndc_product_code": [
"00013-2626"
],
"affected_product_name": "Genotropin",
"affected_product_ingredient": {
"drugbank_id": "DB00052",
"name": "Somatotropin"
},
"description": "The therapeutic efficacy of Somatotropin can be decreased when used in combination with Estradiol.",
"extended_description": "Estrogens affects the actions of growth hormone (GH) at early age by stimulating pituitary GH secretion, modulating GHR-JAK2-STAT5 signalling pathway, ...",
"action": "decrease_therapeutic_efficacy",
"severity": "moderate",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_2",
"management": "Monitor for reduced growth hormone efficacy..."
}
],
"total_results": 1
}
Return a list of drug interactions involving the products with the DPD IDs
02341093and02282623:
curl -L 'https://api.drugbank.com/v1/ddi?dpd=02341093,02282623'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"interactions": [
{
"product_dpd_ids": [
"02341093"
],
"product_name": "Accel-amlodipine",
"product_ingredient": {
"drugbank_id": "DB00381",
"name": "Amlodipine"
},
"affected_product_dpd_id": [
"02282623"
],
"affected_product_name": "Act Risperidone",
"affected_product_ingredient": {
"drugbank_id": "DB00734",
"name": "Risperidone"
},
"description": "Amlodipine may increase the hypotensive activities of Risperidone.",
"extended_description": "Risperidone may induce orthostatic hypotension associated with dizziness, tachycardia, and in some patients, syncope, especially during the initial dos...",
"action": "increase_specific_effects",
"severity": "minor",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_1",
"management": "Caution should be exercised when co-administering risperidone with agents associated with hypotension..."
}
],
"total_results": 1
}
HTTP Request
GET https://api.drugbank.com/v1/ddi?<PRODUCT_ID_FIELD>=<LOCAL_PRODUCT_ID>,<LOCAL_PRODUCT_ID>
Query Parameters
| Parameter | Description |
|---|---|
<PRODUCT_ID_FIELD> |
Product ID field from corresponding regions |
<LOCAL_PRODUCT_ID> |
A comma delimited list of Local Product IDs |
Searching for local region codes outside of the specified regions is not supported.
Find DDI with Product Concepts
Return a list of drug interactions involving the Product concepts with the DrugBank IDs
DBPC0248838andDBPC0061207:
curl -L 'https://api.drugbank.com/v1/ddi?product_concept_id=DBPC0248838,DBPC0061207'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"interactions": [
{
"product_concept_id": "DBPC0061207",
"product_concept_name": "Methylphenidate 18 mg Tablet, extended release",
"product_ingredient": {
"drugbank_id": "DB00422",
"name": "Methylphenidate"
},
"affected_product_concept_id": "DBPC0248838",
"affected_product_concept_name": "Isoflurane 100% [Forane]",
"affected_product_ingredient": {
"drugbank_id": "DB00753",
"name": "Isoflurane"
},
"description": "Methylphenidate may increase the hypertensive activities of Isoflurane.",
"extended_description": "Prescribing information for many formulations of methylphenidate recommend against the use of methylphenidate with a halogenated anesthetic...",
"action": "increase_specific_effects",
"severity": "major",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_1",
"management": "The combined use of methylphenidate with a halogenated anesthetic should be avoided..."
}
],
"total_results": 1
}
HTTP Request
GET https://api.drugbank.com/v1/ddi
Query Parameters
| Parameter | Description |
|---|---|
| product_concept_id | A comma delimited list of DrugBank Product concept IDs. |
To search by Product concept the Product concept IDs should be joined with a comma, and not include any quotations.
Find DDI with DrugBank ID
Return a list of drug interactions involving drug ingredients with the DrugBank IDs
DB09034andDB00615:
curl -L 'https://api.drugbank.com/v1/ddi?drugbank_id=DB00497,DB01193'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"interactions": [
{
"ingredient": {
"drugbank_id": "DB01193",
"name": "Acebutolol"
},
"affected_ingredient": {
"drugbank_id": "DB00497",
"name": "Oxycodone"
},
"description": "The metabolism of Oxycodone can be decreased when combined with Acebutolol.",
"extended_description": "The subject drug is known to be an inhibitor of CYP2D6 while the affected drug is reported to be metabolized by CYP2D6...",
"action": "decrease_dynamics",
"severity": "moderate",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_2",
"management": "If concomitant therapy is required, patients should be monitored for adverse effects related to the affected drug..."
}
],
"total_results": 1
}
HTTP Request
GET https://api.drugbank.com/v1/ddi
Query Parameters
| Parameter | Description |
|---|---|
| drugbank_id | A comma delimited list of DrugBank drug IDs. |
To search by DrugBank ID the DrugBank IDs should be joined with a comma, and not include any quotations.
Find DDI with mixed input
Return a list of drug interactions involving the specified products, drugs, and/or product concepts:
curl -L 'https://api.drugbank.com/v1/ddi?ndc=0143-9503,0056-0173&drugbank_id=DB00503,DB01221,DB00331,DB00819&product_concept_id=DBPC0024484,DBPC0085378'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"interactions": [
{
"product_concept_id": "DBPC0024484",
"product_concept_name": "Abacavir",
"product_ingredient": {
"drugbank_id": "DB01048",
"name": "Abacavir"
},
"affected_product_ndc_product_code": [
"00056-0173"
],
"affected_product_name": "Coumadin",
"affected_product_ingredient": {
"drugbank_id": "DB00682",
"name": "Warfarin"
},
"description": "Abacavir may decrease the excretion rate of Warfarin which could result in a higher serum level.",
"extended_description": "The renal excretion of drugs is the overall result of a combination of kidney processes that include glomerular filtration, passive diffusion, tubular ...",
"action": "decrease_excretion",
"severity": "moderate",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_2",
"management": "Refer to individual drug prescribing information for dosing or management guidelines..."
},
{
"product_concept_id": "DBPC0024484",
"product_concept_name": "Abacavir",
"product_ingredient": {
"drugbank_id": "DB01048",
"name": "Abacavir"
},
"affected_ingredient": {
"drugbank_id": "DB01221",
"name": "Ketamine"
},
"description": "Abacavir may decrease the excretion rate of Ketamine which could result in a higher serum level.",
"extended_description": "The renal excretion of drugs is the overall result of a combination of kidney processes that include glomerular filtration, passive diffusion, tubular ...",
"action": "decrease_excretion",
"severity": "minor",
"subject_dosage": "",
"affected_dosage": "",
"evidence_level": "level_2",
"management": "Refer to individual drug prescribing information for dosing or management guidelines. Caution is generally advised."
},
"..."
],
"total_results": 28
}
The interacting ingredients in each product are identified by name and DrugBank identifier.
HTTP Request
POST https://api.drugbank.com/v1/ddi
To search with multiple IDs/terms, combine the paramaters as specified in the examples above into a single request. Interactions returned by this method may be drug-drug, drug-product, drug-product-concept, etc. The output format therefore can vary from interaction to interaction, depending on what parameters it is based on.
Indications
DrugBank’s structured indications provide a method of describing the uses of a drugs. Documentation is available for the structured indications and conditions objects.
Common Query Parameter Values
When specified, the parameters listed in the table below can be used with the following values:
| Parameter | Value | Description |
|---|---|---|
| more | null | No effect - indications are returned based solely on the names of conditions. |
| more | specific | Include indications for more specific forms of the conditions matching the q parameter. |
| more | general | Include indications for more general forms of the conditions matching the q parameter. |
| off_label | null | No effect - indications will be returned regardless of off_label value. |
| off_label | false | Only labelled indications will be returned. |
| off_label | true | Only off-label indications will be returned. |
| kind | blank | Indications will be returned regardless of kind attribute. |
| kind | single value | Example: kind=treatment_of Only indications for which the kind attribute is “treatment_of” will be returned. |
| kind | comma-delimted values | Example: kind=treatment_of,management_of Only indications for which the kind attribute matches the provided values will be returned. |
| otc_use | null | No effect - indications will be returned regardless of otc_use value. |
| otc_use | true | Only indications for which the otc_use attribute is true will be returned. |
| otc_use | false | Only indications for which the otc_use attribute is false will be returned. |
Data Types
The indications API uses the following data types:
Structured Indications
{
"off_label": false,
"otc_use": false,
"adjunct_use": false,
"military_use": false,
"kind": "used_in_combination_as_diagnostic_agent",
"condition": {
"title": "occlusive vascular disease",
"meddra_id": null,
"icd10_id": null
},
"process": {
"title": "magnetic resonance angiography",
"meddra_id": "llt/10057784",
"icd10_id": null
},
"min_age": {
"amount": 10,
"unit": "day"
},
"max_age": {
"amount": 20,
"unit": "year"
},
"with_therapies": [{
"title": "Prostate external beam radiation therapy",
"meddra_id": "llt/10036924",
"icd10_id": null
}],
"with_drugs": [
{
"name": "Flutamide",
"drugbank_id": "DB00499",
"combination_type": "mixture"
}
],
"with_drug_categories": [
{
"title": "Corticosteroids",
"drugbank_id": "DBCAT000428",
"mesh_id": "D000305"
}
],
"combination_type": "mixture"
}
Note that the above indication includes extra data for the purpose of illustrating the related data structures.
| Property | Type | Description |
|---|---|---|
| off_label | boolean | |
| otc_use | boolean | |
| adjunct_use | boolean | When accessing via a product or product concept, true if the dbpc is directly related to the indication, otherwise false. |
| kind | string | Denotes the kind of use this indication describes. |
| timeline | string | Specifies the timeline (ex. short-term), if necessary. |
| route | string | Route of administration of the drug(s) in the indication. |
| dose_form | string | Dose form in which the indication applies. |
| dose_strength | string | Dose strength in which the indication applies. |
| regions | string array | A list of regions to which this indication applies. |
| sex_group | string | Denotes any restrictions of this indication based on patient sex. Possible values are null, “male”, “female”, or “all”. |
| min_age | object | This indication applies only to patients this age or older. |
| max_age | object | This indication applies only to patients this age or younger. |
| age_groups | string array | List of age groups to which this indication applies. |
| excluded_age_groups | string array | List of age groups to which this indication does not apply. |
| genetic_factors | Genetic Factor array | |
| genetic_factors.includes | Genetic Factor array | |
| genetic_factors.excludes | Genetic Factor array | |
| combination_drugs | simplified Drug array | Denotes drugs administered in combination for this indication. |
| adjunct_drugs | simplified Drug array | Denotes drugs administered in adjunct for this indication. |
| combination_drug_categories | simplified Category array | Denotes drug categories administered in combination for this indication. |
| adjunct_drug_categories | simplified Category array | Denotes drug categories administered in adjunct for this indication. |
| combination_type | string | Describes how the indication combines drugs. |
Some indication properties contain lists of simple objects, described in the table below:
| Property | Array? | Item Properties |
|---|---|---|
| combination_drugs | array | drugbank_id, name |
| adjunct_drugs | array | drugbank_id, name |
| combination_drug_categories | array | drugbank_id, title, mesh_id |
| adjunct_drug_categories | array | drugbank_id, title, mesh_id |
| min_age | object | amount (integer), unit (string, one of “day”, “week”, “month”, “year”) |
| max_age | object | amount (integer), unit (string, one of “day”, “week”, “month”, “year”) |
Within indications, it is commonly required to describe a medical condition. These are described below, in the condition objects section. The following indication properties take this form:
| Property | Type | Description |
|---|---|---|
| condition | Condition | The condition which is the target of this indication. |
| induction_of | Condition | A condition which is induced by the drug. |
| process | Condition | Denotes a diagnosic process in diagnost indications. |
| therapy | Condition | Describes a therapy which is provided by this drug. |
| mechanism | Condition | Denotes the mechanism through which this drug achieves/contributes to the desired change. |
| condition_associated_with | Condition array | |
| equivalent_concept | Condition | A condition equivalent to the indication. Some indications can be directly mapped to MedDRA or ICD10 concepts. |
| with_therapies | Condition array | Denotes adjunct therapies in adjunct indications. |
| patient_characteristics | Condition array | List of one or more patient characteristics. Example: “immunocompromised” |
| excluded_patient_characteristic | Condition array | List of characteristics which exclude patients from this indication. |
combination_type
Combination indications include a combination_type field which denotes the type of combination described. This field can take on one of the 3 values listed in the table below.
| combination_type Value | Description |
|---|---|
| product | Indication refers to a single product with multiple ingredients |
| mixture | Indication refers to many products which use this ingredient along with others. In those combination products, each ingredient may have a distinct purpose. Example: cough/cold products often combine many ingredients, each with a separate purpose. |
| regimen | Indication refers to a drug (potentially in a combination product) which is used with other products as part of a treatment regimen or cocktail. |
Some indications apply to a combination product being used as part of a regimen. These indications will have a "combination_type": "regimen" value, which may obscure the fact that some of the drugs in with_drugs are used in a combination product with the indicated drug. For this reason, each drug in the with_drugs array is given its own combination_type field. Generally this matches the combination_type of the enclosing indication, but in the product/regimen scenario, any of the drugs in with_drugs with "combination_type": "product" are part of the combination product including the main drug of this indication.
Genetic Factors
Describes a genetic factor for indications, or conditions.
| Property | Type | Description |
|---|---|---|
| gene | Condition | |
| mutation | Condition | |
| positive_finding | Condition | |
| negative_finding | Condition | |
| related_genes | Condition array |
Search indications
curl -L 'https://api.drugbank.com/v1/indications?q=arthritis'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"kind": "used_in_combination_for_symptomatic_treatment_of",
"off_label": false,
"otc_use": true,
"route": [
"Topical"
],
"dose_form": [],
"dose_strength": [],
"combination_type": "product",
"drug": {
"name": "Menthyl salicylate",
"drugbank_id": "DB11201"
},
"regions": "US",
"condition": {
"name": "Arthritis",
"drugbank_id": "DBCOND0016433",
"meddra_id": "pt/10003246",
"snomed_id": "d/7278014",
"icd10_id": "c/M19.90"
},
"combination_drugs": [
{
"name": "Levomenthol",
"drugbank_id": "DB00825"
}
]
}
]
This endpoint searches for conditions, and then returns indications related to those conditions. Documentation is available for the indication and condition JSON objects.
This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/indications
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | “” | Text used to search conditions by name. |
| more | null | Determines how to broaden the condition search results. The original results will be included regardless of the value of this parameter. |
| off_label | null | Limits results by the value of the off_label attribute of the indications. |
| otc_use | null | Limits results by the value of the otc_use attribute of the indications. |
| kind | null | Limits results by the value of the kind attribute of the indications. |
Get Drugs By Indications Search
curl -L 'https://api.drugbank.com/v1/indications/drugs?q=arthritis'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DB11201",
"name": "Menthyl salicylate",
"cas_number": "89-46-3",
"annotation_status": "complete",
"availability_by_region": [
{
"region": "ca",
"max_phase": 4,
"marketed_prescription": false,
"generic_available": false,
"pre_market_cancelled": false,
"post_market_cancelled": true
},
{
"region": "us",
"max_phase": 4,
"marketed_prescription": false,
"generic_available": false,
"pre_market_cancelled": true,
"post_market_cancelled": false
},
"..."
],
"description": "Menthyl salicylate is an ester of menthol and salicylic acid [L2808]...",
"simple_description": null,
"clinical_description": null,
"synonyms": [
"1-Menthyl salicylate",
"Menthyl salicylate, (+/-)-"
],
"pharmacology": {
"indication_description": "\r\n\r\nFor the temporary relief of pain associated with rheumatism, arthritis, neuralgia, sprains and strains of joints and muscles, lumbago and fibrositi...",
"pharmacodynamic_description": "Menthol and methyl salicylate are known as counterirritants...",
"mechanism_of_action_description": "\r\nMenthol dilates the blood vessels causing a sensation of coldness, followed by an analgesic effect...",
"absorption": "May be absorbed through intact skin...",
"protein_binding": "",
"volume_of_distribution": [],
"clearance": [],
"half_life": "",
"route_of_elimination": "",
"toxicity_description": "Salicylate intoxication may occur after ingestion or topical application of menthyl salicylate..."
},
"food_interactions": [],
"identifiers": {
"drugbank_id": "DB11201",
"inchi": "InChI=1S/C17H24O3/c1-11(2)13-9-8-12(3)10-16(13)20-17(19)14-6-4-5-7-15(14)18/h4-7,11-13,16,18H,8-10H2,1-3H3/t12-,13+,16-/m1/s1",
"inchikey": "SJOXEWUZWQYCGL-DVOMOZLQSA-N",
"atc_codes": []
},
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000049",
"name": "Anti-Inflammatory Agents, Non-Steroidal",
"mesh_id": "D000894",
"mesh_tree_numbers": [
"D27.505.696.663.850.014.040.500",
"D27.505.954.158.030",
"..."
],
"atc_code": "S01BC",
"atc_level": 4,
"synonyms": [
"Agents, Aspirin-Like",
"Agents, Non-Steroidal Anti-Inflammatory",
"..."
],
"description": "Anti-inflammatory agents that are non-steroidal in nature..."
}
]
}
]
This endpoint retrieves drugs based on a search of conditions and related indications. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/indications/drugs
This endpoint supports pagination.
URL Parameters
| Parameter | Default | Description |
|---|---|---|
| q | “” | Text used to search conditions by name. |
| more | null | Determines how to broaden the condition search results. The original results will be included regardless of the value of this parameter. |
| off_label | null | Limits results by the value of the off_label attribute of the indications. |
| otc_use | null | Limits results by the value of the otc_use attribute of the indications. |
| kind | null | Limits results by the value of the kind attribute of the indications. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| include_references | false | If true, includes the lists of references for each drug. See References for details. |
Example: Search indications by condition and more specific forms
curl -L 'https://api.drugbank.com/v1/indications/drugs?q=autoimmune+disorders&more=specific'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DB00531",
"name": "Cyclophosphamide",
"cas_number": "50-18-0",
"annotation_status": "complete",
"availability_by_region": [
{
"region": "ca",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": false,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
{
"region": "us",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
"..."
],
"description": "Precursor of an alkylating nitrogen mustard antineoplastic and immunosuppressive agent that must be activated in the liver to form the active aldophosp...",
"simple_description": "A medication used to treat certain cancers.",
"clinical_description": "A nitrogen mustard used to treat lymphomas, myelomas, leukemia, mycosis fungoides, neuroblastoma, ovarian adenocarcinoma, retinoblastoma, and breast ca...",
"synonyms": [
"(+-)-Cyclophosphamide",
"(±)-2-(BIS(2-CHLOROETHYL)AMINO)TETRAHYDRO-2H-1,3,2-OXAZAPHOSPHORINE 2-OXIDE MONOHYDRATE",
"..."
],
"pharmacology": {
"indication_description": "Cyclophosphamide for intravenous injection is indicated for the treatment of a number of malignancies, including: Hodgkin’s disease, lymphocytic lympho...",
"pharmacodynamic_description": "Cyclophosphamide is an antineoplastic in the class of alkylating agents and is used to treat various forms of cancer...",
"mechanism_of_action_description": "Alkylating agents work by three different mechanisms: 1) attachment of alkyl groups to DNA bases, resulting in the DNA being fragmented by repair enzym...",
"absorption": "After oral administration, peak concentrations occur at one hour. ",
"protein_binding": "20% of cyclophosphamide is protein bound with no dose dependent changes. Some metabolites are protein bound to an extent greater than 60%. ",
"volume_of_distribution": [
"30-50 L"
],
"clearance": [
"Total body clearance = 63 ± 7.6 L/kg."
],
"half_life": "3-12 hours",
"route_of_elimination": "Cyclophosphamide is eliminated primarily in the form of metabolites...",
"toxicity_description": "Adverse reactions reported most often include neutropenia, febrile neutropenia, fever, alopecia, nausea, vomiting, and diarrhea.\r\n"
},
"food_interactions": [
"Drink plenty of fluids. Drink 2 to 3 liters of fluids a day.",
"Take with food. Food reduces irritation."
],
"identifiers": {
"drugbank_id": "DB00531",
"inchi": "InChI=1S/C7H15Cl2N2O2P/c8-2-5-11(6-3-9)14(12)10-4-1-7-13-14/h1-7H2,(H,10,12)",
"inchikey": "CMSMOCZEIVJLDB-UHFFFAOYSA-N",
"atc_codes": [
{
"code": "L01AA01",
"title": "cyclophosphamide",
"combination": false
}
]
},
"therapeutic_categories": [
{
"drugbank_id": "DBCAT003311",
"name": "Alkylating Drugs",
"mesh_id": "D000477",
"mesh_tree_numbers": [
"D27.505.519.124",
"D27.888.569.035"
],
"atc_code": "L01A",
"atc_level": 3,
"synonyms": [
"Agents, Alkylating",
"Alkylating Agent",
"..."
],
"description": "Highly reactive chemicals that introduce alkyl radicals into biologically active molecules and thereby prevent their proper functioning..."
}
]
}
]
HTTP Request
GET https://api.drugbank.com/v1/indications?q=autoimmune+disorders&more=specific
Gets indications for items that match the search term, as well as any more specific terms. For instance, this could match indications for ‘autoimmune disorders’, as well as ‘rheumatoid arthritis’, ‘Celiac disease’, etc..
Conditions
Common Query Parameter Values
When specified, the parameters listed in the table below can be used with the following values:
| Parameter | Value | Description |
|---|---|---|
| more | null | No effect - indications are returned based solely on the names of conditions. |
| more | specific | Include indications for more specific forms of the conditions matching the q parameter. |
| more | general | Include indications for more general forms of the conditions matching the q parameter. |
Data Types
The conditions API uses the following data types:
Conditions
{
"title": "magnetic resonance angiography",
"drugbank_id": "DBCOND0000008",
"meddra_id": "llt/10057784",
"icd10_id": null,
"snomed_id": null,
"related_concepts": [{
"title": "magnetic resonance imaging",
"drugbank_id": "DBCOND0000007",
"meddra_id": null,
"icd10_id": null
}]
}
Condition objects with title, meddra_id, icd10_id, and snomed_id properties are used in several places in structured indication objects. Often, condition objects denote a medical condition, but they are also used to describe procedures, therapies, and genes.
Condition objects have the following properties
| Property | Type | Description |
|---|---|---|
| title | string | Name/description of the condition |
| drugbank_id | string | DrugBank id for the product concept. |
| meddra_id | string | MedDRA identifier |
| icd10_id | string | ICD10 identifier |
| snomed_id | string | SNOMED identifier |
| uniprot_id | string | UniProt identifer, for conditions which describe a gene. |
| related_concepts | Condition array | |
| as_drug | Drug | The DrugBank drug of the same name as this condition, if any exists. |
| as_drug_category | Category | The DrugBank drug category of the same name as this condition, if any exists. |
| modification_of | Object | See below |
| combination_of | Object | See below |
combination_of
{
"title": "Conjunctivitis caused by chlamydia",
"drugbank_id": "DBCOND0021916",
"meddra_id": "pt/10010745",
"icd10_id": "c/A74.0",
"synonyms": [
"Conjunctivitis chlamydial",
"Chlamydial Conjunctivitis",
"Inclusion blenorrhoea",
"Inclusion blenorrhea",
"Conjunctivitis, Inclusion",
"Paratrachoma",
"Inclusion conjunctivitis"
],
"combination_of": {
"additional_characteristics": [],
"caused_by": [
{
"title": "Chlamydial Infections",
"drugbank_id": "DBCOND0020995",
"meddra_id": "hlt/10008561",
"icd10_id": "c/A74.9",
"uniprot_id": null
}
],
"included_conditions": [
{
"title": "Conjunctivitis",
"drugbank_id": "DBCOND0009989",
"meddra_id": "llt/10010741",
"icd10_id": "c/H10.9",
"uniprot_id": null
}
],
"excluded_conditions": []
}
}
This property describes the outer Condition as a combination of other conditions. Often, this is used to tie a group of conditions to each individual condition which are more likely to have a MedDRA ID, ICD10 ID, etc.
| Property | Type | Description |
|---|---|---|
| caused_by | Condition array | |
| included_conditions | Condition array | |
| excluded_conditions | Condition array | |
| additional_characteristics | Condition array | Characteristic of the combination condition. |
modification_of
{
"title": "acute, non-severe allergic rhinitis",
"modification_of": {
"base": {
"title": "allergic rhinitis"
},
"severity": {
"includes": [ "acute" ],
"excludes": [ "severe" ]
}
}
}
This property describes the outer Condition as a modification to another condition. Often, this is used to tie a slightly more specific form of a condition to a more general form, which is more likely to have a MedDRA ID, ICD10 ID, etc. For instance, “acute allergic rhinitis” could be considered to be “allergic rhinitis” with the additional modifier “acute”.
| Property | Type | Description |
|---|---|---|
| base | Condition | The base condition which is being modified. |
| location | string | |
| condition_stage | string | |
| condition_status | string | |
| severity | object | |
| severity.includes | string array | |
| severity.excludes | string array |
Condition Search Plugin
Our condition search plugin provides a “plug and play” alternative to implementing our search conditions endpoint:
As a standard web component, it is easy to integrate and style to suit your needs. For more information, please see this public repository.
Search conditions
curl -L 'https://api.drugbank.com/v1/conditions?q=arth'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"hits": [
{
"field": "general title",
"value": "<em>Arth</em>ritis/<em>Arth</em>rosis"
},
{
"field": "title",
"value": "<em>Arth</em>ritis/<em>Arth</em>rosis"
}
],
"name": "Arthritis/Arthrosis",
"drugbank_id": "DBCOND0086825",
"uniprot_id": null,
"meddra_id": null,
"icd10_id": null,
"snomed_id": "d/400379010"
},
{
"hits": [
{
"field": "general title",
"value": "<em>Arth</em>ritis and <em>Arth</em>ralgia"
},
{
"field": "title",
"value": "<em>Arth</em>ritis and <em>Arth</em>ralgia"
}
],
"name": "Arthritis and Arthralgia",
"drugbank_id": "DBCOND0142150",
"uniprot_id": null,
"meddra_id": null,
"icd10_id": null,
"snomed_id": null
},
"..."
]
This endpoint returns a list of conditions suitable for use with autocomplete forms based on a query string. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/conditions
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | “” | Text used to search conditions by name. |
| fuzzy | false | If set to true, enable fuzzy search (see fuzzy searching). |
| exact | false | Determines how text results are matched. exact=true will not include partial matches such as ‘Rheumatoid Arthritis’ for the query ‘arthritis’. |
Get a condition
This endpoint retrieves a specific condition based on ID (DrugBank Condition ID).
curl -L 'https://api.drugbank.com/v1/conditions/DBCOND0015777'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"name": "Gout",
"drugbank_id": "DBCOND0015777",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10",
"modification_of": {
"base": {
"name": "Gouty Arthritis",
"drugbank_id": "DBCOND0052747",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10"
},
"severity": {
"includes": [
"acute"
],
"excludes": []
}
},
"more_general": [
{
"name": "Disorders of purine metabolism",
"drugbank_id": "DBCOND0027454",
"meddra_id": "hlt/10070968",
"snomed_id": "d/54443017"
},
{
"name": "Purine and pyrimidine metabolism disorders",
"drugbank_id": "DBCOND0027729",
"meddra_id": "hlgt/10037546",
"snomed_id": "d/3036760010",
"icd10_id": "c/E79.9"
},
"..."
],
"more_specific": [
{
"name": "Refractory Gout",
"drugbank_id": "DBCOND0025787",
"meddra_id": "llt/10088490",
"snomed_id": "c/90560007",
"icd10_id": "c/M10.9"
},
{
"name": "Chronic, refractory Gout",
"drugbank_id": "DBCOND0112054",
"icd10_id": "c/M1A.9"
},
"..."
],
"indications": [
{
"kind": "adjunct_therapy_in_treatment_of",
"off_label": false,
"otc_use": false,
"route": [
"Intramuscular",
"Subcutaneous"
],
"dose_form": [
"Gel",
"Injection",
"..."
],
"dose_strength": [],
"timeline": "short-term",
"drug": {
"name": "Corticotropin",
"drugbank_id": "DB01285"
},
"regions": "US",
"condition": {
"name": "Acute Gouty Arthritis",
"drugbank_id": "DBCOND0032167",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10",
"modification_of": {
"base": {
"name": "Gouty Arthritis",
"drugbank_id": "DBCOND0052747",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10"
},
"severity": {
"includes": [
"acute"
],
"excludes": []
}
}
},
"patient_characteristics": [
{
"name": "Acute Exacerbation",
"drugbank_id": "DBCOND0034186",
"modification_of": {
"base": {
"name": "Exacerbation",
"drugbank_id": "DBCOND0036928"
},
"severity": {
"includes": [
"acute"
],
"excludes": []
}
}
}
]
},
{
"kind": "adjunct_therapy_in_management_of",
"off_label": false,
"otc_use": false,
"route": [
"Intra-articular",
"Intramuscular",
"..."
],
"dose_form": [
"Injection",
"Tablet"
],
"dose_strength": [],
"drug": {
"name": "Methylprednisolone",
"drugbank_id": "DB00959"
},
"regions": "US",
"condition": {
"name": "Acute Gouty Arthritis",
"drugbank_id": "DBCOND0032167",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10",
"modification_of": {
"base": {
"name": "Gouty Arthritis",
"drugbank_id": "DBCOND0052747",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10"
},
"severity": {
"includes": [
"acute"
],
"excludes": []
}
}
}
},
"..."
]
}
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Get indications linked to a condition
curl -L 'https://api.drugbank.com/v1/conditions/DBCOND0015777/indications'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"kind": "adjunct_therapy_in_treatment_of",
"off_label": false,
"otc_use": false,
"route": [
"Intramuscular",
"Subcutaneous"
],
"dose_form": [
"Gel",
"Injection",
"..."
],
"dose_strength": [],
"timeline": "short-term",
"drug": {
"name": "Corticotropin",
"drugbank_id": "DB01285"
},
"regions": "US",
"condition": {
"name": "Acute Gouty Arthritis",
"drugbank_id": "DBCOND0032167",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10",
"modification_of": {
"base": {
"name": "Gouty Arthritis",
"drugbank_id": "DBCOND0052747",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10"
},
"severity": {
"includes": [
"acute"
],
"excludes": []
}
}
},
"patient_characteristics": [
{
"name": "Acute Exacerbation",
"drugbank_id": "DBCOND0034186",
"modification_of": {
"base": {
"name": "Exacerbation",
"drugbank_id": "DBCOND0036928"
},
"severity": {
"includes": [
"acute"
],
"excludes": []
}
}
}
]
},
{
"kind": "adjunct_therapy_in_management_of",
"off_label": false,
"otc_use": false,
"route": [
"Intra-articular",
"Intramuscular",
"..."
],
"dose_form": [
"Injection",
"Tablet"
],
"dose_strength": [],
"drug": {
"name": "Methylprednisolone",
"drugbank_id": "DB00959"
},
"regions": "US",
"condition": {
"name": "Acute Gouty Arthritis",
"drugbank_id": "DBCOND0032167",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10",
"modification_of": {
"base": {
"name": "Gouty Arthritis",
"drugbank_id": "DBCOND0052747",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10"
},
"severity": {
"includes": [
"acute"
],
"excludes": []
}
}
}
},
"..."
]
This endpoint retrieves indications linked to a condition.
HTTP Request
GET https://api.drugbank.com/v1/conditions/<ID>/indications
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| more | null | Determines how to broaden the condition search results. The original results will be included regardless of the value of this parameter. |
| off_label | null | Limits results by the value of the off_label attribute of the indications. |
| otc_use | null | Limits results by the value of the otc_use attribute of the indications. |
| kind | null | Limits results by the value of the kind attribute of the indications. |
This endpoint supports pagination.
Get product concepts linked to a condition
curl -L 'https://api.drugbank.com/v1/conditions/DBCOND0015777/product_concepts'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Corticotropin Intramuscular; Subcutaneous Injection",
"display_name": null,
"drugbank_pcid": "DBPC0217426",
"brand": null,
"level": 3,
"route": "Intramuscular; Subcutaneous",
"form": "Injection",
"strengths": null,
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2005-09-09",
"regions": {
"us": true,
"canada": false,
"eu": false,
"germany": false,
"colombia": false,
"turkey": false,
"italy": false,
"malaysia": false,
"austria": false,
"indonesia": false,
"thailand": false,
"singapore": false,
"mexico": false
},
"rxnorm_concepts": [
{
"name": "corticotropin Injectable Solution",
"RXCUI": "371633"
}
],
"ingredients": [
{
"name": "Corticotropin",
"drug": {
"name": "Corticotropin",
"drugbank_id": "DB01285"
}
}
]
},
{
"name": "Methylprednisolone",
"display_name": null,
"drugbank_pcid": "DBPC0017364",
"brand": null,
"level": 1,
"route": null,
"form": null,
"strengths": null,
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1957-10-24",
"regions": {
"us": true,
"canada": true,
"eu": false,
"germany": true,
"colombia": true,
"turkey": true,
"italy": true,
"malaysia": true,
"austria": true,
"indonesia": true,
"thailand": true,
"singapore": true,
"mexico": true
},
"rxnorm_concepts": [
{
"name": "methylprednisolone",
"RXCUI": "6902"
}
],
"ingredients": [
{
"name": "Methylprednisolone",
"drug": {
"name": "Methylprednisolone",
"drugbank_id": "DB00959"
}
}
]
},
"..."
]
This endpoint retrieves product concepts linked to a condition.
HTTP Request
GET https://api.drugbank.com/v1/conditions/<ID>/product_concepts
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| more | null | Determines how to broaden the condition search results. The original results will be included regardless of the value of this parameter. |
| off_label | null | Limits results by the value of the off_label attribute of the indications used to find product concepts. |
| otc_use | null | Limits results by the value of the otc_use attribute of the indications used to find product concepts. |
| kind | null | Limits results by the value of the kind attribute of the indications used to find product concepts. |
| level | The product concept level to filter by (optional). | |
| min_level | The minimum product concept level to return (optional). | |
| min_level | The maximum product concept level to return (optional). | |
| unbranded_only | false | If true, returns only product concepts without an associated brand. |
Get drugs linked to a condition via indications
curl -L 'https://api.drugbank.com/v1/conditions/DBCOND0012160/drugs'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DB01551",
"name": "Dihydrocodeine",
"cas_number": "125-28-0",
"annotation_status": "complete",
"availability_by_region": [
{
"region": "ca",
"max_phase": 0,
"marketed_prescription": false,
"generic_available": false,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
{
"region": "us",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
"..."
],
"description": "Dihydrocodeine is an opioid analgesic used as an alternative or adjunct to codeine to treat moderate to severe pain, severe dyspnea, and cough...",
"simple_description": "A medication used to reduce severe pain in various conditions, including surgeries and dental procedures.",
"clinical_description": "An opioid analgesic agent used for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequ...",
"synonyms": [
"DHC",
"Dihydrocodeine"
],
"pharmacology": {
"indication_description": "Dihydrocodeine is used for the treatment of moderate to severe pain, including post-operative and dental pain [2]...",
"pharmacodynamic_description": "Possible opioid related side effects include, but are not limited to, drowsiness, nausea, headache, dry mouth, constipation, difficulty passing urine, ...",
"mechanism_of_action_description": "Dihydrocodeine is metabolized to dihydromorphine -- a highly active metabolite with a high affinity for mu opioid receptors. [3]",
"absorption": "Bioavailability is low (approximately 20%) if administered orally...",
"protein_binding": "",
"volume_of_distribution": [
"The disposition of dihydrocodeine is described as a two compartment model. [2]"
],
"clearance": [
"Plasma clearance is approximately 300ml/min. [2]",
"",
"..."
],
"half_life": "4h",
"route_of_elimination": "Renal elimination and urinary excretion. [1]",
"toxicity_description": ""
},
"food_interactions": [
"Avoid alcohol. Alcohol can enhance the CNS depressant effects of this drug."
],
"identifiers": {
"drugbank_id": "DB01551",
"inchi": "InChI=1S/C18H23NO3/c1-19-8-7-18-11-4-5-13(20)17(18)22-16-14(21-2)6-3-10(15(16)18)9-12(11)19/h3,6,11-13,17,20H,4-5,7-9H2,1-2H3/t11-,12+,13-,17-,18-/m0/s...",
"inchikey": "RBOXVHNMENFORY-DNJOTXNNSA-N",
"atc_codes": [
{
"code": "N02AA58",
"title": "dihydrocodeine, combinations",
"combination": true
},
{
"code": "N02AA08",
"title": "dihydrocodeine",
"combination": false
},
"..."
]
},
"therapeutic_categories": [
{
"drugbank_id": "DBCAT003293",
"name": "Opioid Agonist",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Opioid Agonists"
],
"description": null
}
]
},
{
"drugbank_id": "DB00945",
"name": "Acetylsalicylic acid",
"cas_number": "50-78-2",
"annotation_status": "complete",
"availability_by_region": [
{
"region": "ca",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
{
"region": "us",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
"..."
],
"description": "Also known as _Aspirin_, acetylsalicylic acid (ASA) is a commonly used drug for the treatment of pain and fever due to various causes...",
"simple_description": "A painkiller used to treat pain, fever, inflammation, and migraines, as well as reduce the risk of heart attacks, strokes, and other events.",
"clinical_description": "A salicylate used to treat pain, fever, inflammation, migraines, and reducing the risk of major adverse cardiovascular events.",
"synonyms": [
"2-Acetoxybenzenecarboxylic acid",
"2-Acetoxybenzoic acid",
"..."
],
"pharmacology": {
"indication_description": "**Pain, fever, and inflammation**\r\n\r\nAcetylsalicylic acid (ASA), in the regular tablet form (immediate-release), is indicated to relieve pain, fever, a...",
"pharmacodynamic_description": "**Effects on pain and fever**\r\n\r\nAcetylsalicylic acid disrupts the production of prostaglandins throughout the body by targeting cyclooxygenase-1 (COX-...",
"mechanism_of_action_description": "Acetylsalicylic acid (ASA) blocks prostaglandin synthesis...",
"absorption": "\r\nAbsorption is generally rapid and complete following oral administration but absorption may be variable depending on the route, dosage form, and othe...",
"protein_binding": "50% to 90% of a normal therapeutic concentration salicylate (a main metabolite of acetylsalicylic acid [FDA label]) binds plasma proteins, particularly...",
"volume_of_distribution": [
"This drug is distributed to body tissues shortly after administration..."
],
"clearance": [
"The clearance rate of acetylsalicylic acid is extremely variable, depending on several factors [A177217]..."
],
"half_life": "The half-life of ASA in the circulation ranges from 13 - 19 minutes...",
"route_of_elimination": "Excretion of salicylates occurs mainly through the kidney, by the processes of glomerular filtration and tubular excretion, in the form of free salicyl...",
"toxicity_description": "**Lethal doses**\r\n\r\nAcute oral LD50 values have been reported as over 1..."
},
"food_interactions": [
"Avoid alcohol. Alcohol increases the risk of gastrointestinal bleeding.",
"Avoid herbs and supplements with anticoagulant/antiplatelet activity...",
"..."
],
"identifiers": {
"drugbank_id": "DB00945",
"inchi": "InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12)",
"inchikey": "BSYNRYMUTXBXSQ-UHFFFAOYSA-N",
"atc_codes": [
{
"code": "B01AC06",
"title": "acetylsalicylic acid",
"combination": false
},
{
"code": "C07FX04",
"title": "bisoprolol and acetylsalicylic acid",
"combination": true
},
"..."
]
},
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000049",
"name": "Anti-Inflammatory Agents, Non-Steroidal",
"mesh_id": "D000894",
"mesh_tree_numbers": [
"D27.505.696.663.850.014.040.500",
"D27.505.954.158.030",
"..."
],
"atc_code": "S01BC",
"atc_level": 4,
"synonyms": [
"Agents, Aspirin-Like",
"Agents, Non-Steroidal Anti-Inflammatory",
"..."
],
"description": "Anti-inflammatory agents that are non-steroidal in nature..."
},
{
"drugbank_id": "DBCAT000149",
"name": "Antiplatelet agents",
"mesh_id": "D010975",
"mesh_tree_numbers": [
"D27.505.954.502.780"
],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Agents with Antiplatelet Properties",
"Agents with Antiplatelet Properties (e.g., P2Y12 inhibitors, NSAIDs, SSRIs, etc.)",
"..."
],
"description": "Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape chang..."
},
"..."
]
},
"..."
]
This endpoint retrieves drugs linked to a condition via indications.
HTTP Request
GET https://api.drugbank.com/v1/conditions/<ID>/drugs
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| more | null | Determines how to broaden the condition search results. The original results will be included regardless of the value of this parameter. |
| off_label | null | Limits results by the value of the off_label attribute of the indications used to find drugs. |
| otc_use | null | Limits results by the value of the otc_use attribute of the indications used to find drugs. |
| kind | null | Limits results by the value of the kind attribute of the indications used to find drugs. |
| include_references | false | If true, includes the lists of references for each drug. See References for details. |
Get more general forms of a condition
curl -L 'https://api.drugbank.com/v1/conditions/DBCOND0015777/general'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Disorders of purine metabolism",
"drugbank_id": "DBCOND0027454",
"meddra_id": "hlt/10070968",
"snomed_id": "d/54443017"
},
{
"name": "Purine and pyrimidine metabolism disorders",
"drugbank_id": "DBCOND0027729",
"meddra_id": "hlgt/10037546",
"snomed_id": "d/3036760010",
"icd10_id": "c/E79.9"
},
"..."
]
This endpoint retrieves more general forms of a condition.
HTTP Request
GET https://api.drugbank.com/v1/conditions/<ID>/general
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Get more specific forms of a condition
curl -L 'https://api.drugbank.com/v1/conditions/DBCOND0015777/specific'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Refractory Gout",
"drugbank_id": "DBCOND0025787",
"meddra_id": "llt/10088490",
"snomed_id": "c/90560007",
"icd10_id": "c/M10.9",
"modification_of": {
"base": {
"name": "Gout",
"drugbank_id": "DBCOND0015777",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10"
},
"severity": {
"includes": [
"refractory"
],
"excludes": []
}
},
"combination_of": {
"additional_characteristics": [
"Refractory"
],
"included_conditions": [
{
"name": "Gout",
"drugbank_id": "DBCOND0015777",
"meddra_id": "llt/10018634",
"snomed_id": "d/767712010",
"icd10_id": "c/M10"
}
]
}
},
{
"name": "Refractory Chronic Gout",
"drugbank_id": "DBCOND0116735",
"icd10_id": "c/M1A.9",
"modification_of": {
"base": {
"name": "Refractory Gout",
"drugbank_id": "DBCOND0025787",
"meddra_id": "llt/10088490",
"snomed_id": "c/90560007",
"icd10_id": "c/M10.9"
},
"severity": {
"includes": [
"chronic"
],
"excludes": []
}
}
},
"..."
]
This endpoint retrieves more specific forms of a condition.
HTTP Request
GET https://api.drugbank.com/v1/conditions/<ID>/specific
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Get related conditions
curl -L 'https://api.drugbank.com/v1/conditions/DBCOND0023821/references'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Inflammation",
"drugbank_id": "DBCOND0012215",
"meddra_id": "llt/10021995",
"snomed_id": "d/39611018"
},
{
"name": "Pain",
"drugbank_id": "DBCOND0012160",
"meddra_id": "llt/10033371",
"snomed_id": "d/253262010",
"icd10_id": "c/R52"
}
]
This endpoint retrieves related conditions.
HTTP Request
GET https://api.drugbank.com/v1/conditions/<ID>/references
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Allergies
View Allergy Alternatives for a Drug
curl -L 'https://api.drugbank.com/v1/drugs/DB09156/allergy_alternatives'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DBCAT003315",
"name": "Radiographic Contrast Agent",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"categorization_kind": "therapeutic",
"synonyms": [
"Radiographic Contrast Agents",
"Radiographic Contrast Drug",
"..."
],
"description": null,
"drugs": [
{
"name": "Ioxilan",
"drugbank_id": "DB09135",
"cross_sensitivities": [
{
"summary": "As an Iodine Radio-contrast Medium, Iopromide is known to be cross-sensitive with Iodine Radio-contrast Media",
"incidence": "67%"
}
]
},
{
"name": "Diatrizoate",
"drugbank_id": "DB00271",
"cross_sensitivities": [
{
"summary": "As an Iodine Radio-contrast Medium, Iopromide is known to be cross-sensitive with Iodine Radio-contrast Media",
"incidence": "67%"
}
]
},
"..."
]
}
]
This endpoint retrieves lists of drugs as allergy alternatives based on the provided drug’s therapeutic categorizations and cross-sensitivities.
The results are returned as individual categories where the provided drug is considered therapeutic for that category, each with a separate list of drug alternatives. Any applicable cross-sensitivities are shown with summary and incidence.
For more information on a specific cross-sensitivity, use this endpoint with the applicable DrugBank IDs.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/allergy_alternatives
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the drug alternatives. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| show_cross_sensitivities | true | When set to false, alternatives with any cross sensitivities will be filtered out. When set to true, all alternatives will be shown with inline cross sensitivity information. |
View Drug Alternatives for a Product Allergy
curl -L 'https://api.drugbank.com/v1/products/57841-1150/product_concepts/allergy_alternatives'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Omeprazole",
"drugbank_id": "DB00338",
"categories": [
{
"drugbank_id": "DBCAT000541",
"name": "Proton Pump Inhibitors",
"mesh_id": "D054328",
"mesh_tree_numbers": [
"D27.505.519.389.848"
],
"atc_code": "A02BC",
"atc_level": 4,
"categorization_kinds": [
"therapeutic",
"pharmacological",
"..."
],
"synonyms": [
"Inhibitors, Proton Pump",
"PP Inhibitors",
"..."
],
"description": "Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE...",
"drugs": [
{
"name": "Pantoprazole",
"drugbank_id": "DB00213",
"cross_sensitivities": [
{
"summary": "As a Proton Pump Inhibitor, Omeprazole is known to be cross-sensitive with Proton Pump Inhibitors",
"incidence": "Rare"
}
]
},
{
"name": "Lansoprazole",
"drugbank_id": "DB00448",
"cross_sensitivities": [
{
"summary": "As a Proton Pump Inhibitor, Omeprazole is known to be cross-sensitive with Proton Pump Inhibitors",
"incidence": "Rare"
}
]
},
"..."
]
},
{
"drugbank_id": "DBCAT003638",
"name": "Proton-pump Inhibitors",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"categorization_kinds": [
"therapeutic"
],
"synonyms": [],
"description": null,
"drugs": [
{
"name": "Pantoprazole",
"drugbank_id": "DB00213",
"cross_sensitivities": [
{
"summary": "As a Proton Pump Inhibitor, Omeprazole is known to be cross-sensitive with Proton Pump Inhibitors",
"incidence": "Rare"
}
]
},
{
"name": "Lansoprazole",
"drugbank_id": "DB00448",
"cross_sensitivities": [
{
"summary": "As a Proton Pump Inhibitor, Omeprazole is known to be cross-sensitive with Proton Pump Inhibitors",
"incidence": "Rare"
}
]
},
"..."
]
}
]
}
]
This endpoint retrieves lists of drugs as allergy alternatives based on the therapeutic categorizations of the ingredients associated with the provided product.
Related products to the drug alternatives can then be found using this endpoint.
For more information on a specific cross-sensitivity, use this endpoint with the applicable DrugBank IDs.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/product_concepts/allergy_alternatives
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the linked drug alternatives. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| show_cross_sensitivities | true | When set to false, alternatives with any cross sensitivities will be filtered out. When set to true, all alternatives will be shown with inline cross sensitivity information. |
View Drug Alternatives for a Product Concept Allergy
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC0011501/allergy_alternatives'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"name": "Hydrochlorothiazide",
"drugbank_id": "DB00999",
"categories": [
{
"drugbank_id": "DBCAT005157",
"name": "Antihypertensive Agents Indicated for Hypertension",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"categorization_kinds": [
"therapeutic"
],
"synonyms": [],
"description": "Included in this category are agents that have approved indications for the treatment of hypertension...",
"drugs": [
{
"name": "Acebutolol",
"drugbank_id": "DB01193",
"cross_sensitivities": []
},
{
"name": "Aliskiren",
"drugbank_id": "DB09026",
"cross_sensitivities": []
},
"..."
]
},
{
"drugbank_id": "DBCAT000542",
"name": "Diuretics",
"mesh_id": "D004232",
"mesh_tree_numbers": [
"D27.505.696.560.500"
],
"atc_code": "C03",
"atc_level": 2,
"categorization_kinds": [
"therapeutic",
"pharmacological",
"..."
],
"synonyms": [
"Diuretic Effect",
"Diuretic Effects",
"..."
],
"description": "Agents that promote the excretion of urine through their effects on kidney function.",
"drugs": [
{
"name": "Torasemide",
"drugbank_id": "DB00214",
"cross_sensitivities": []
},
{
"name": "Etacrynic acid",
"drugbank_id": "DB00903",
"cross_sensitivities": []
},
"..."
]
},
"..."
]
}
]
This endpoint retrieves lists of drugs as allergy alternatives based on the therapeutic categorizations of the ingredients associated with the provided product concept.
Related product concepts to the drug alternatives can then be found using this endpoint.
For more information on a specific cross-sensitivity, use this endpoint with the applicable DrugBank IDs.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/allergy_alternatives
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve the linked drug alternatives. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| show_cross_sensitivities | true | When set to false, alternatives with any cross sensitivities will be filtered out. When set to true, all alternatives will be shown with inline cross sensitivity information. |
Drug-Allergy Checker
curl -L 'https://api.drugbank.com/v1/cross_sensitivities?known_drugbank_id=DB01060&drugbank_id=DB01326'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"ingredient": {
"name": "Amoxicillin",
"drugbank_id": "DB01060"
},
"cross_sensitive_entries": [
{
"ingredient": {
"name": "Cefamandole",
"drugbank_id": "DB01326"
}
}
],
"summary": "As a Penicillin, Amoxicillin is known to be cross-sensitive with Cephalosporins",
"description": "Recent studies suggest that cross-sensitivity between penicillins and cephalosporins arise from the similarity between the R1 side chain of earlier gen...",
"incidence": "<10%",
"evidence_type": [
"uncontrolled_trial",
"review"
]
}
]
This endpoint checks known drug allergies against a list of drugs, products or product concepts (including mixed input) for possible cross-sensitivities.
HTTP Request
GET https://api.drugbank.com/v1/cross_sensitivities/
URL Parameters
| Parameter | Description |
|---|---|
| known_drugbank_id | A comma delimited list of DrugBank IDs for which an allergy is known. |
| known_product_concept_id | A comma delimited list of DrugBank Product Concept IDs for which an allergy is known. |
| known_LOCAL_PRODUCT_ID | A comma delimited list of Percent encoded Local Product IDs for which an allergy is known. |
| drugbank_id | A comma delimited list of DrugBank IDs to check against. |
| product_concept_id | A comma delimited list of DrugBank Product Concept IDs to check against. |
| LOCAL_PRODUCT_ID | A comma delimited list of Percent encoded Local Product IDs to check against. |
| include_references | If true, includes the lists of references for cross-sensitivity information (defaults to false). See References for details. |
Allergy Presentation Search
curl -L 'https://api.drugbank.com/v1/allergy_presentations?q=anaph'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"hits": [
{
"field": "title",
"value": "<em>Anaph</em>ylaxis"
},
{
"field": "synonyms",
"value": "<em>anaph</em>ylactic"
}
],
"drugbank_id": "DBCOND0001820",
"title": "Anaphylaxis"
},
{
"hits": [
{
"field": "title",
"value": "Nonimmunologic <em>Anaph</em>ylaxis"
},
{
"field": "synonyms",
"value": "Shock <em>anaph</em>ylactic <em>anaph</em>ylactoid"
}
],
"drugbank_id": "DBCOND0131490",
"title": "Nonimmunologic Anaphylaxis"
},
"..."
]
This endpoint searches for conditions related to allergic presentations. It is compatible with autocomplete and fuzzy search.
HTTP Request
GET https://api.drugbank.com/v1/allergy_presentations/
URL Parameters
| Parameter | Default | Description |
|---|---|---|
| q | null | The string you want to search with. |
| fuzzy | false | If set to true, enable fuzzy search (see Fuzzy Searching). |
Notice the hits array returned in the results. The hits contain highlighted
snippets from the match. You can use these highlights in autocomplete applications.
The matching part of the text is wrapped in an <em> tag, which you can style
as you wish in your application.
Get a Presentation
curl -L 'https://api.drugbank.com/v1/allergy_presentations/DBCOND0001820'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"condition": {
"drugbank_id": "DBCOND0001820",
"title": "Anaphylaxis"
},
"hypersensitivity_types": [
"Type I"
],
"simple_description": "Anaphylaxis is a life-threatening allergic reaction that can cause low blood pressure, difficulty breathing, and a skin rash.",
"clinical_description": "Anaphylaxis is an allergic reaction that causes urticaria, dyspnea, wheezing, nausea, vomiting, syncope, and hypotension...",
"management": "Anaphylaxis is a life-threatening condition and is generally treated with prompt withdrawal of the offending medication, early intramuscular or subcuta..."
}
This endpoint retrieves a specific presentation entry based on its condition ID.
HTTP Request
GET https://api.drugbank.com/v1/allergy_presentations/<ID>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The condition ID of the presentation to retrieve. |
Allergy Presentation Checker
curl -L 'https://api.drugbank.com/v1/allergy_checker?condition_id=DBCOND0029210&drugbank_id=DB01326,DB00316&product_concept_id=DBPC0725449'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"allergic_presentations": [
{
"name": "Hypersensitivity Vasculitis",
"drugbank_id": "DBCOND0029210",
"meddra_id": "llt/10020764",
"snomed_id": "d/100609019",
"icd10_id": "c/M31.0",
"possible_causative_entries": [
{
"ingredient": {
"name": "Cefamandole",
"drugbank_id": "DB01326"
}
},
{
"product_concept": {
"name": "Tivozanib 1.34 mg Capsule",
"product_concept_id": "DBPC0725449"
},
"ingredient": {
"name": "Tivozanib",
"drugbank_id": "DB11800"
}
}
]
}
]
}
This endpoint checks a list of drugs, products or product concepts (including mixed input) to identify possible causes for an allergy presentation.
HTTP Request
GET https://api.drugbank.com/v1/allergy_checker/
URL Parameters
| Parameter | Description |
|---|---|
| condition_id | A comma delimited list of condition IDs to check against. |
| drugbank_id | A comma delimited list of DrugBank IDs. |
| product_concept_id | A comma delimited list of DrugBank Product Concept IDs. |
| LOCAL_PRODUCT_ID | A comma delimited list of Percent encoded Local Product IDs. |
Common query parameter values for allergy detail and cross sensitivity endpoints
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| include_presentation_details | false | If set to true, include details of each presentation. |
| include_source_details | false | If set to true, include source_name and source_type for this allergy detail (useful when there are multiple allergy details). |
| include_references | false | If true, includes the lists of references for allergy information. See References for details. |
Get allergy details for a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB09156/allergy_details'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"summary": "Hypersensitivity to Iopromide can present as: Stevens-Johnson Syndrome Toxic Epidermal Necrolysis Spectrum, Allergy-Induced Respiratory Symptoms, Angio...",
"info": "Hypersensitivity reactions to iopromide tend to be rare in clinical practice...",
"evidence_type": [
"case_reports",
"review"
],
"hypersensitivity_types": [
"Type I",
"Type IV",
"..."
],
"presentations": [
"Allergy-Induced Respiratory Symptoms",
"Anaphylaxis",
"..."
]
}
]
This endpoint retrieves allergy information linked to a drug.
Details will be given as a list which may be empty if the drug has no associated allergy information.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/allergy_details
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the allergy details. |
Get allergy details linked with a product
curl -L 'https://api.drugbank.com/v1/products/57841-1150/allergy_details'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"ingredient": {
"name": "Rifabutin",
"drugbank_id": "DB00615"
},
"details": [
{
"summary": "Hypersensitivity to Rifamycins can present as: Cutaneous Manifestations of Drug Allergy, drug-induced lupus erythematosus, Allergic Glomerulonephritis,...",
"info": "Rifamycins are members of the ansamycin antibiotic family with an ansa poly-hydroxylated bridge connecting both ends of a naphthoquinone core, and are ...",
"evidence_type": [
"clinical_trial",
"post_marketing",
"..."
],
"hypersensitivity_types": [
"Type I",
"Type II",
"..."
],
"presentations": [
"Acute Generalized Exanthematous Pustulosis",
"Acute Interstitial Nephritis",
"..."
]
}
]
}
]
This endpoint retrieves allergy information linked to ingredients in a product.
Ingredients with no associated allergy details will not show up. Allergy details for each ingredient will show up as a list.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/allergy_details
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the allergy details. |
Get allergy details linked with a product concept
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC503562/allergy_details'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Amoxicillin",
"drugbank_id": "DB01060"
},
"details": [
{
"summary": "Hypersensitivity to Penicillins can present as: Cutaneous Manifestations of Drug Allergy and Erythema multiforme",
"info": "Penicillin allergy is the most common drug allergy, with incidence ranging between five and 25% by geographical region and population...",
"evidence_type": [
"case_reports",
"review"
],
"hypersensitivity_types": [
"Type IV",
"Unclassified"
],
"presentations": [
"Cutaneous Manifestations of Drug Allergy",
"Erythema multiforme"
]
}
]
}
]
This endpoint retrieves allergy information linked to components of a product concept.
Components with no associated allergy details will not show up. Allergy details for each component will show up as a list.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/allergy_details
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve the allergy details. |
Get cross-sensitivities for a drug
curl -L 'https://api.drugbank.com/v1/drugs/DB09156/cross_sensitivities'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"cross_sensitive_drugs": [
{
"name": "Acetrizoic acid",
"drugbank_id": "DB09347"
},
{
"name": "Diatrizoate",
"drugbank_id": "DB00271"
},
"..."
],
"summary": "As an Iodine Radio-contrast Medium, Iopromide is known to be cross-sensitive with Iodine Radio-contrast Media",
"description": "Iodine radio-contrast media hypersensitivity reactions may be due to broadly sensitive T cells, according to lymphocyte transferase tests...",
"incidence": "67%",
"evidence_type": [
"case_reports",
"review"
]
}
]
This endpoint retrieves cross-sensitivity information linked to a drug.
Cross-sensitivities will be given as a list which may be empty if the drug has no associated cross-sensitivities.
HTTP Request
GET https://api.drugbank.com/v1/drugs/<ID>/cross_sensitivities
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the drug to retrieve the cross-sensitivities. |
Get cross-sensitivities linked with a product
curl -L 'https://api.drugbank.com/v1/products/57841-1150/cross_sensitivities'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"ingredient": {
"name": "Rifabutin",
"drugbank_id": "DB00615"
},
"cross_sensitive_drugs": [
{
"name": "Rifampin",
"drugbank_id": "DB01045"
},
{
"name": "Rifamycin",
"drugbank_id": "DB11753"
},
"..."
],
"summary": "As a Rifamycin, Rifabutin is known to be cross-sensitive with Rifamycins",
"description": "One _in vitro_ study was performed to determine cross-sensitivity in a patient who was hypersensitive to rifamycin SV and rifaximin...",
"incidence": "Rare",
"evidence_type": [
"case_reports"
]
}
]
This endpoint retrieves cross-sensitivity information linked to ingredients in a product.
Ingredients with no associated cross-sensitivities will not show up. Cross-sensitivities for each ingredient will show up as a list.
HTTP Request
GET https://api.drugbank.com/v1/products/<LOCAL_PRODUCT_ID>/cross_sensitivities
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | Percent encoded Local Product ID of the product to retrieve the cross-sensitivities. |
Get cross-sensitivities linked with a product concept
curl -L 'https://api.drugbank.com/v1/product_concepts/DBPC503562/cross_sensitivities'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Amoxicillin",
"drugbank_id": "DB01060"
},
"cross_sensitive_drugs": [
{
"name": "Clavulanic acid",
"drugbank_id": "DB00766"
}
],
"summary": "As a Penicillin, Amoxicillin is known to be cross-sensitive with Clavulanic acid",
"description": "An allergy to clavulanic acid may lead to an allergy to other beta-lactams, such as penicillins and cephalosporins, due to similarities in chemical str...",
"incidence": "Theoretical",
"evidence_type": [
"review"
]
}
]
This endpoint retrieves cross-sensitivity information linked to components of a product concept.
Components with no associated cross-sensitivities will not show up. Cross-sensitivities for each component will show up as a list.
HTTP Request
GET https://api.drugbank.com/v1/product_concepts/<ID>/cross_sensitivities
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the product concept to retrieve the cross-sensitivities. |
ICD-10
ICD-10 Search Plugin
Our ICD-10 search plugin provides a “plug and play” alternative to implementing our ICD-10 search endpoint:
As a standard web component, it is easy to integrate and style to suit your needs. For more information, please see this public repository.
ICD-10 Search
curl -L 'https://api.drugbank.com/v1/icd10?q=ameb'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"hits": [
{
"field": "description",
"value": "<em>Ameb</em>iasis"
}
],
"identifier": "A06",
"description": "Amebiasis"
},
{
"hits": [
{
"field": "description",
"value": "<em>Ameb</em>ic cystitis"
}
],
"identifier": "A06.81",
"description": "Amebic cystitis"
},
"..."
]
This endpoint searches for ICD-10 concepts: either using the ICD-10 code itself, or by the concept description or inclusion terms. This search is compatible with autocomplete forms and fuzzy search.
HTTP Request
GET https://api.drugbank.com/v1/icd10
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | null | The string you want to search with. |
| fuzzy | false | If set to true, enable fuzzy search (see Fuzzy Searching). |
Notice the hits array returned in the results. The hits contain highlighted
snippets from the match. You can use these highlights in autocomplete applications.
The matching part of the text is wrapped in an <em> tag, which you can style
as you wish in your application. Note that while the hits array includes matches
to inclusion terms, the ICD-10 concepts returned are limited to the identifier and
description.
Categories
Common Category Query Parameter Values
When specified, the parameters listed in the table below can be used with the following values:
| Parameter | Value | Description |
|---|---|---|
| include_children | blank | No effect. |
| include_children | 1, 2 or 3 | Include nested children to a depth of 1, 2, or 3. |
| include_parents | blank | No effect. |
| include_parents | 1, 2 or 3 | Include nested parents to a depth of 1, 2, or 3. |
| source | blank or all |
Include children/parents from any hierarchy. |
| source | atc |
Include children/parents from the ATC hierarchy. |
| source | mesh |
Include children/parents from the MeSH hierarchy. |
To limit the amount of data included, parent/child inclusion only goes outward
from the categories being shown. I.E. include_parents=all&include_children=all
will show all children and all parents of a category, not all children and all
parents of all categories related categories. In other words,
"parents": [] will not include "children": [] and vice-versa.
ATC and MeSH identifiers
Categories with equivalent entries in ATC and MeSH can be accessed by the identifiers used in ATC and MeSH, in addition to the DrugBank id assigned to those categories. In most cases below, examples of requests by ATC or MeSH identifier will be given without the response, as this would be the regardless of which identifier is used.
View Category
curl -L 'https://api.drugbank.com/v1/categories/DBCAT001220'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"drugbank_id": "DBCAT001034",
"name": "Xanthine derivatives",
"mesh_id": "D013806",
"mesh_tree_numbers": [
"D03.132.960.751",
"D03.633.100.759.758.824.751"
],
"atc_code": "N06BC",
"atc_level": 4,
"categorization_kinds": [
"therapeutic",
"pharmacological",
"..."
],
"synonyms": [
"1,3 Dimethylxanthine",
"1,3-Dimethylxanthine",
"..."
],
"description": "A methyl xanthine derivative from tea with diuretic, smooth muscle relaxant, bronchial dilation, cardiac and central nervous system stimulant activitie..."
}
This endpoint retrieves a specific category based on DrugBank ID, ATC Code, or MeSH ID.
HTTP Request
GET https://api.drugbank.com/v1/categories/<ID>
GET https://api.drugbank.com/v1/categories/atc/<ATC_CODE>
GET https://api.drugbank.com/v1/categories/mesh/<MESH_ID>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the category. |
| ATC_CODE | The ATC Code of the category. |
| MESH_ID | The unique MeSH identifier of the category. |
View Category by ATC code
curl -L 'https://api.drugbank.com/v1/categories/atc/L01'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"drugbank_id": "DBCAT000024",
"name": "ANTINEOPLASTIC AGENTS",
"mesh_id": "D000970",
"mesh_tree_numbers": [
"D27.505.954.248"
],
"atc_code": "L01",
"atc_level": 2,
"categorization_kinds": [
"pharmacological",
"indexing"
],
"synonyms": [
"Agents, Anticancer",
"Agents, Antineoplastic",
"..."
],
"description": "Substances that inhibit or prevent the proliferation of NEOPLASMS."
}
This endpoint retrieves a specific category based on ATC Code.
HTTP Requests
GET https://api.drugbank.com/v1/categories/atc/<ATC_CODE>
URL Parameters
| Parameter | Description |
|---|---|
| ATC_CODE | The ATC Code of the category to retrieve the parent categories. |
View Category by MeSH Unique ID
curl -L 'https://api.drugbank.com/v1/categories/mesh/D015122'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"drugbank_id": null,
"name": "Mercaptopurine",
"mesh_id": "D015122",
"mesh_tree_numbers": [
"D02.886.489.534",
"D03.633.100.759.570"
],
"atc_code": null,
"atc_level": null,
"categorization_kinds": [],
"synonyms": [],
"description": null
}
This endpoint retrieves a specific category based on MeSH Unique Identifier.
HTTP Requests
GET https://api.drugbank.com/v1/categories/mesh/<MESH_ID>/parents
URL Parameters
| Parameter | Description |
|---|---|
| MESH_ID | The unique MeSH identifier of the category to retrieve the parent categories. |
View Parents of a Category
curl -L 'https://api.drugbank.com/v1/categories/DBCAT002744/parents?source=mesh&include_parents=2'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DBCAT000499",
"name": "Erythromycin",
"mesh_id": "D004917",
"mesh_tree_numbers": [
"D02.540.576.500.992"
],
"atc_code": "S01AA17",
"atc_level": 5,
"categorization_kinds": [
"indexing"
],
"synonyms": [
"C, Erythromycin",
"Erycette",
"..."
],
"description": "A bacteriostatic antibiotic macrolide produced by Streptomyces erythreus...",
"parents": [
{
"drugbank_id": "DBCAT000496",
"name": "Macrolides",
"mesh_id": "D018942",
"mesh_tree_numbers": [
"D02.540.505",
"D02.540.576.500",
"..."
],
"atc_code": "J01FA",
"atc_level": 4,
"synonyms": [
"Antibiotic, Macrolide",
"Macrolide Antibiotics",
"..."
],
"description": "A group of often glycosylated macrocyclic compounds formed by chain extension of multiple PROPIONATES cyclized into a large (typically 12, 14, or 16)-m...",
"parents": [
{
"drugbank_id": "DBCAT000498",
"name": "Lactones",
"mesh_id": "D007783",
"mesh_tree_numbers": [
"D02.540"
],
"atc_code": null,
"atc_level": null,
"synonyms": [],
"description": "Cyclic esters of hydroxy carboxylic acids, containing a 1-oxacycloalkan-2-one structure. Large cyclic lactones of over a dozen atoms are MACROLIDES."
},
{
"drugbank_id": "DBCAT000497",
"name": "Polyketides",
"mesh_id": "D061065",
"mesh_tree_numbers": [
"D02.540.576",
"D04.345.674"
],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Ketides"
],
"description": "Natural compounds containing alternating carbonyl and methylene groups (beta-polyketones), bioenergenetically derived from repeated condensation of ace..."
}
]
}
]
}
]
Returns an array of categories which are parents of the specified category.
Accepts the source parameter to limit relationships.
By default this will return all parents.
This endpoint supports pagination.
HTTP Requests
GET https://api.drugbank.com/v1/categories/<ID>/parents
GET https://api.drugbank.com/v1/categories/atc/<ATC_CODE>/parents
GET https://api.drugbank.com/v1/categories/mesh/<MESH_ID>/parents
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the category to retrieve the child categories. |
| ATC_CODE | The ATC Code of the category to retrieve the child categories. |
| MESH_ID | The unique MeSH identifier of the category to retrieve the child categories. |
View Children of a Category
curl -L 'https://api.drugbank.com/v1/categories/DBCAT000522/children'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DBCAT000944",
"name": "Anthelmintics",
"mesh_id": "D000871",
"mesh_tree_numbers": [
"D27.505.954.122.250.075"
],
"atc_code": "P02",
"atc_level": 2,
"categorization_kinds": [
"therapeutic",
"pharmacological",
"..."
],
"synonyms": [
"Antihelmintics",
"Vermifuges"
],
"description": "Agents destructive to parasitic worms. They are used therapeutically in the treatment of HELMINTHIASIS in man and animal."
},
{
"drugbank_id": "DBCAT002211",
"name": "Antiprotozoal Agents",
"mesh_id": "D000981",
"mesh_tree_numbers": [
"D27.505.954.122.250.100"
],
"atc_code": "P01",
"atc_level": 2,
"categorization_kinds": [
"therapeutic",
"pharmacological",
"..."
],
"synonyms": [
"Agents Against Protozoal Diseases",
"Agents, Antiprotozoal",
"..."
],
"description": "Substances that are destructive to protozoans."
}
]
Returns an array of categories which are children of the specified category.
Accepts the source parameter to limit relationships.
By default this will return all parents.
This endpoint supports pagination.
HTTP Requests
GET https://api.drugbank.com/v1/categories/<ID>/children
GET https://api.drugbank.com/v1/categories/atc/<ATC_CODE>/children
GET https://api.drugbank.com/v1/categories/mesh/<MESH_ID>/children
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the category to retrieve the child categories. |
| ATC_CODE | The ATC Code of the category to retrieve the child categories. |
| MESH_ID | The unique MeSH identifier of the category to retrieve the child categories. |
Get Drugs in a Category
curl -L 'https://api.drugbank.com/v1/categories/DBCAT001220/drugs'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DB01303",
"name": "Oxtriphylline",
"cas_number": "4499-40-5",
"annotation_status": "complete",
"availability_by_region": [
{
"region": "ca",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": true,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
{
"region": "us",
"max_phase": 0,
"marketed_prescription": false,
"generic_available": false,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
"..."
],
"description": "Oxtriphylline is the choline salt form of [theophylline]...",
"simple_description": "A medication used to treat conditions in the lungs causing difficulty breathing, such as asthma and inflammation.",
"clinical_description": "A bronchodilator used for the treatment of asthma, bronchitis, COPD, and emphysema.",
"synonyms": [
"Choline theophyllinate",
"Choline theophylline",
"..."
],
"pharmacology": {
"indication_description": "Used to treat the symptoms of asthma, bronchitis, COPD, and emphysema.",
"pharmacodynamic_description": "Oxtriphylline is a bronchodilator...",
"mechanism_of_action_description": "Oxtriphylline is a choline salt of theophylline...",
"absorption": "After ingestion, theophylline is released from oxytriphylline in the acidic environment of the stomach. ",
"protein_binding": "With a serum concentrations of 17 mcg/mL, adults and children have about 56% theophylline bound to plasma protein, and premature infants have about 36%...",
"volume_of_distribution": [
"Theophylline has an apparent volume of distribution of 0..."
],
"clearance": [
"Theophylline has an average clearance in children (over 6 months) of 1.45 mL/kg per minute, and in healthy, nonsmoking adults of 0.65 mL/kg per hour."
],
"half_life": "The serum half life varies greatly between patients and in age...",
"route_of_elimination": "The kidneys are the main route of elimination for both theophylline and its metabolites, but some unchanged theophylline is eliminated in the feces.",
"toxicity_description": "Symptoms of toxicity include abdominal pain (continuing or severe), confusion or change in behavior, convulsions (seizures), dark or bloody vomit, diar..."
},
"food_interactions": [
"Limit caffeine intake.",
"Take with food. Food reduces irritation."
],
"identifiers": {
"drugbank_id": "DB01303",
"inchi": "InChI=1S/C7H8N4O2.C5H14NO/c1-10-5-4(8-3-9-5)6(12)11(2)7(10)13;1-6(2,3)4-5-7/h3H,1-2H3,(H,8,9,12);7H,4-5H2,1-3H3/q;+1/p-1",
"inchikey": "RLANKEDHRWMNRO-UHFFFAOYSA-M",
"atc_codes": [
{
"code": "R03DA20",
"title": "combinations of xanthines",
"combination": true
},
{
"code": "R03DB02",
"title": "choline theophyllinate and adrenergics",
"combination": true
},
"..."
]
},
"therapeutic_categories": [
{
"drugbank_id": "DBCAT003651",
"name": "Respiratory Smooth Muscle Relaxants",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"synonyms": [],
"description": null
}
]
}
]
Returns all drugs in this category.
This endpoint supports pagination.
HTTP Requests
GET https://api.drugbank.com/v1/categories/<ID>/drugs
GET https://api.drugbank.com/v1/categories/atc/<ATC_CODE>/drugs
GET https://api.drugbank.com/v1/categories/mesh/<MESH_ID>/drugs
URL Parameters
| Parameter | Description |
|---|---|
| ID | The DrugBank ID of the category to retrieve the linked drugs. |
| ATC_CODE | The ATC Code of the category to retrieve the linked drugs. |
| MESH_ID | The unique MeSH identifier of the category to retrieve the linked drugs. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| include_references | false | If true, includes the lists of references for each drug. See References for details. |
Search Category Names / Autocomplete
curl -L 'https://api.drugbank.com/v1/category_names?q=antimetabolites'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"categories": [
{
"hits": [
{
"field": "name",
"value": "<em>Antimetabolites</em>"
}
],
"drugbank_id": "DBCAT000276",
"name": "Antimetabolites",
"mesh_id": "D000963",
"mesh_tree_numbers": [
"D27.505.519.186",
"D27.888.569.042"
],
"atc_code": "L01B",
"atc_level": 3,
"categorization_kinds": [
"therapeutic",
"pharmacological",
"..."
],
"synonyms": [],
"description": "Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways..."
}
]
}
This endpoint returns a list of categories suitable for use with autocomplete forms, for quickly finding the right categories. Note that this search is for DrugBank categories and will not include results for ATC or Mesh categories that don’t map to a corresponding DrugBank category.
This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/category_names
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | null | The string you want to search with. |
| fuzzy | false | If set to true, enable fuzzy search (see fuzzy searching below). |
Notice the hits array returned in the results. The hits contain highlighted
snippets from the match. You can use these highlights in autocomplete applications.
The matching part of the text is wrapped in an <em> tag, which you can style
as you wish in your application.
Fuzzy Searching
This example demonstrates a misspelling of “Antimetabolite”, with fuzzy search enabled you will still get a result (try it yourself!).
curl -L 'https://api.drugbank.com/v1/category_names?q=aantimetabolites&fuzzy=true'
-H 'Authorization: myapikey'
Fuzzy searching allows for misspellings, but is not enabled by default,
you must set fuzzy=true. By setting fuzzy=true you are telling the API to
allow a certain number of misspellings to still count as a match (defaults to 2).
You can also pass a number of misspellings (0, 1, or 2) in to tailor the
likelihood of a match (for example, fuzzy=1 will allow 1 misspelled letter).
Adverse Effects
Common Query Parameter Values
When specified, the parameters listed in the table below can be used with the following values:
| Parameter | Value | Description |
|---|---|---|
| more | null | No effect - adverse effects are returned based solely on the names of conditions. |
| more | specific | Include adverse effects for more specific forms of the conditions matching the q parameter. |
| more | general | Include adverse effects for more general forms of the conditions matching the q parameter. |
Search adverse effects
curl -L 'https://api.drugbank.com/v1/adverse_effects?q=cardiac'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Donislecel",
"drugbank_id": "DB17961"
},
"route": [
"Intravascular"
],
"dose_form": [
"Solution"
],
"dose_strength": [],
"evidence_type": [
"clinical_trial"
],
"trial_name": [],
"regions": "US",
"age_groups": [
"adult"
],
"min_age": {
"amount": 21,
"unit": "year"
},
"max_age": {
"amount": 67,
"unit": "year"
},
"incidences": [
{
"kind": "experimental",
"name": "LANTIDRA",
"percent": "20-90"
}
],
"effect": {
"name": "Palpitations",
"drugbank_id": "DBCOND0007623",
"meddra_id": "pt/10033557",
"snomed_id": "d/252438011",
"icd10_id": "c/R00.2"
},
"patient_characteristics": [
{
"name": "Hypoglycemia Unawareness",
"drugbank_id": "DBCOND0031993",
"meddra_id": "llt/10020997",
"snomed_id": "d/3038723019"
},
{
"name": "Type 1 Diabetes Mellitus",
"drugbank_id": "DBCOND0029465",
"meddra_id": "llt/10045228",
"snomed_id": "d/4571145011",
"icd10_id": "c/E10.8"
}
]
}
]
This endpoint searches for conditions, and then returns adverse effects related to those conditions. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/adverse_effects
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | “” | Text used to search conditions by name. |
| more | null | Determines how to broaden the condition search results. The original results will be included regardless of the value of this parameter. |
| exact | false | Determines how text results are matched. exact=true will not include partial matches such as ‘Rheumatoid Arthritis’ for the query ‘arthritis’. |
Get drugs linked to an adverse effect condition
curl -L 'https://api.drugbank.com/v1/adverse_effects/DBCOND0020133/drugs'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drugbank_id": "DB08911",
"name": "Trametinib",
"cas_number": "871700-17-3",
"annotation_status": "complete",
"availability_by_region": [
{
"region": "ca",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": false,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
{
"region": "us",
"max_phase": 4,
"marketed_prescription": true,
"generic_available": false,
"pre_market_cancelled": false,
"post_market_cancelled": false
},
"..."
],
"description": "Trametinib is an orally bioavailable mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2 inhibitor...",
"simple_description": "A chemotherapy drug used to treat specific types of cancer with certain genetic mutations, such as skin cancer, lung cancer, and thyroid cancer.",
"clinical_description": "A kinase inhibitor used alone or in combination with dabrafenib to treat patients with cancers with specific BRAF mutations, such as melanoma and non-s...",
"synonyms": [
"Tramétinib",
"Trametinib",
"..."
],
"pharmacology": {
"indication_description": "Trametinib is indicated as monotherapy for the treatment of BRAF-inhibitor treatment-naïve patients with unresectable or metastatic melanoma with BRAF ...",
"pharmacodynamic_description": "Trametinib inhibits cell growth of various BRAF V600 mutation-positive tumours _in vitro_ and _in vivo_...",
"mechanism_of_action_description": "The mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK) pathway, also known as the RAS-RAF-MEK-ERK pathway, activates ...",
"absorption": "Following oral administration, trametinib is rapidly and readily absorbed...",
"protein_binding": "Trametinib is 97.4% bound to human plasma proteins.[L45558]",
"volume_of_distribution": [
"The apparent volume of distribution (V<sub>c</sub>/F) is 214 L.[L45558]"
],
"clearance": [
"The apparent clearance is 4.9 L/h.[L45558]"
],
"half_life": "The estimated elimination half-life is 3.9 to 4.8 days.[L45558]",
"route_of_elimination": "Following oral administration of [<sup>14</sup>C]-trametinib, greater than 80% of excreted radioactivity was recovered in the feces while less than 20%...",
"toxicity_description": "There is no information regarding the acute toxicity (LD<sub>50</sub>) of trametinib..."
},
"food_interactions": [
"Take separate from meals..."
],
"identifiers": {
"drugbank_id": "DB08911",
"inchi": "InChI=1S/C26H23FIN5O4/c1-13-22-21(23(31(3)24(13)35)30-20-10-7-15(28)11-19(20)27)25(36)33(17-8-9-17)26(37)32(22)18-6-4-5-16(12-18)29-14(2)34/h4-7,10-12,...",
"inchikey": "LIRYPHYGHXZJBZ-UHFFFAOYSA-N",
"atc_codes": [
{
"code": "L01EE01",
"title": "trametinib",
"combination": false
}
]
},
"therapeutic_categories": [
{
"drugbank_id": "DBCAT003325",
"name": "Kinase Inhibitor",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Kinase Inhibitors"
],
"description": null
}
]
}
]
This endpoint retrieves drugs linked to a condition via adverse effects.
HTTP Request
GET https://api.drugbank.com/v1/adverse_effects/<ID>/drugs
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| more | null | Determines how to broaden the condition search results. The original results will be included regardless of the value of this parameter. |
| include_references | false | If true, includes the lists of references for each drug. See References for details. |
Get products linked to an adverse effect condition
curl -L 'https://api.drugbank.com/v1/adverse_effects/DBCOND0020133/products'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"ndc_product_code": "00338-9572",
"originator_ndc_product_code": "0338-9572",
"dpd_id": null,
"ema_product_code": null,
"ema_ma_number": null,
"pzn": null,
"co_product_id": null,
"tr_product_id": null,
"it_product_id": null,
"my_product_id": null,
"at_product_id": null,
"idn_product_id": null,
"th_product_id": null,
"sg_product_id": null,
"mx_registration_id": null,
"mx_product_id": null,
"name": "Bivalirudin in 0.9% Sodium Chloride",
"prescribable_name": "Bivalirudin 25 mg/5mL Injection",
"rx_norm_prescribable_name": "bivalirudin 250 MG in 50 ML Injection",
"started_marketing_on": "2017-12-21",
"ended_marketing_on": null,
"approved_on": null,
"schedule": null,
"dosage_form": "Injection",
"route": "Intravenous",
"application_number": "NDA208374",
"generic": false,
"otc": false,
"approved": true,
"country": "US",
"mixture": false,
"allergenic": false,
"cosmetic": false,
"vaccine": false,
"ingredients": [
{
"name": "Bivalirudin",
"drugbank_id": "DB00006",
"strength": {
"number": "250",
"unit": "mg/50mL"
}
}
],
"therapeutic_categories": [
{
"drugbank_id": "DBCAT000007",
"name": "Anticoagulants",
"mesh_id": "D000925",
"mesh_tree_numbers": [
"D27.505.954.502.119"
],
"atc_code": null,
"atc_level": null,
"synonyms": [
"Agents, Anticoagulant",
"Anti-coagulant",
"..."
],
"description": "Agents that prevent clotting."
},
{
"drugbank_id": "DBCAT000012",
"name": "Antithrombins",
"mesh_id": "D000991",
"mesh_tree_numbers": [
"D27.505.519.389.745.800.449",
"D27.505.954.502.119.500"
],
"atc_code": "B01AE",
"atc_level": 4,
"synonyms": [
"Antithrombins, Direct",
"Direct Antithrombins",
"..."
],
"description": "Endogenous factors and drugs that directly inhibit the action of THROMBIN, usually by blocking its enzymatic activity..."
}
],
"labeller": {
"name": "Baxter Healthcare Corporation"
},
"images": []
}
]
This endpoint retrieves products linked to a condition via adverse effects.
HTTP Request
GET https://api.drugbank.com/v1/adverse_effects/<ID>/products
URL Parameters
| Parameter | Description |
|---|---|
| ID | The ID of the condition to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| more | null | Determines how to broaden the condition search results. The original results will be included regardless of the value of this parameter. |
| include_simple_desc | false | If set to true, include simple descriptions for the product ingredients. |
| include_clinical_desc | false | If set to true, include clinical descriptions for the product ingredients. |
Boxed Warnings
Search boxed warning
curl -L 'https://api.drugbank.com/v1/boxed_warnings?q=cardiac'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"drug": {
"name": "Abiraterone",
"drugbank_id": "DB05812"
},
"kind": "warning",
"recommendation": "Use caution in patients with a history of cardiovascular disease.",
"lab_values": [],
"route": [
"Oral"
],
"dose_form": [],
"risk": {
"name": "Cardiovascular Events",
"drugbank_id": "DBCOND0048671",
"snomed_id": "d/2157405016",
"icd10_id": "c/I24.9"
}
}
]
This endpoint searches for conditions, and then returns boxed warnings related to those conditions. This endpoint supports pagination.
HTTP Request
GET https://api.drugbank.com/v1/boxed_warnings
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| q | “” | Text used to search conditions by name. |
| exact | false | Determines how text results are matched. exact=true will not include partial matches such as ‘Rheumatoid Arthritis’ for the query ‘arthritis’. |
Packages
Get a specific U.S. package
curl -L 'https://api.drugbank.com/v1/packages/50580-111-08'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"package_ndc_code": "50580-0111-08",
"originator_package_ndc_code": "50580-111-08",
"description": "240 mL LIQUID IN 1 BOTTLE, PLASTIC",
"full_description": "240 mL LIQUID IN 1 BOTTLE, PLASTIC",
"amount": "240",
"unit": "mL",
"form": "BOTTLE, PLASTIC",
"for_product": {
"ndc_product_code": "50580-0111",
"originator_ndc_product_code": "50580-111",
"name": "Tylenol Extra Strength",
"active_ingredients": [
{
"name": "Acetaminophen",
"unii": "362O9ITL9D",
"strength_number": "500",
"strength_unit": "mg/15mL"
}
],
"inactive_ingredients": [
{
"name": "anhydrous citric acid",
"unii": "XF417D3PSL",
"strength_number": null,
"strength_unit": null
},
{
"name": "D&C Red NO. 33",
"unii": "9DBA0SBB0L",
"strength_number": null,
"strength_unit": null
},
"..."
]
},
"packaging_tree": [
{
"package_ndc_code": "50580-0111-08",
"originator_package_ndc_code": "50580-111-08",
"description": "240 mL LIQUID IN 1 BOTTLE, PLASTIC",
"amount": "240",
"unit": "mL",
"form": "BOTTLE, PLASTIC"
}
]
}
Some packages contain multiple levels, shown in the
packaging_treeattribute:
{
"package_ndc_code": "75834-0130-84",
"description": "28 in 1 BLISTER PACK",
"amount": "28",
"unit": "1",
"form": "BLISTER PACK",
"for_product": {
"name": "..."
},
"packaging_tree": [
{
"package_ndc_code": "75834-0130-29",
"description": "3 in 1 CARTON",
"amount": "3",
"unit": "1",
"form": "CARTON",
"content": {
"package_ndc_code": "75834-0130-84",
"description": "28 in 1 BLISTER PACK",
"amount": "28",
"unit": "1",
"form": "BLISTER PACK"
}
}
]
}
Some packages are kits containing multiple parts. In this case the returning JSON will contain a
partsarray:
{
"package_ndc_code": "68405-0018-06",
"description": "1 in 1 KIT",
"amount": "1",
"unit": "1",
"form": "KIT",
"for_product": {
"name": "..."
},
"parts": [
{
"number": 1,
"ndc_product_code": "52959-0190",
"name": "NAPROXEN",
"route": "ORAL",
"amount": "60",
"unit": "1",
"dosage_form": "TABLET",
"marketing_category": "ANDA",
"application_number": "ANDA075927",
"packaging_tree": [
{
"package_ndc_code": "52959-0190-30",
"description": "60 in 1 BOTTLE",
"amount": "60",
"unit": "1",
"form": "BOTTLE"
}
],
"active_ingredients": [
{
"name": "NAPROXEN",
"unii": "57Y76R9ATQ",
"strength_number": "250",
"strength_unit": "mg/1"
}
],
"inactive_ingredients": [
{
"name": "CROSCARMELLOSE SODIUM",
"unii": "M28OL1HH48",
"strength_number": null,
"strength_unit": null
},
{
"name": "POVIDONE",
"unii": "FZ989GH94E",
"strength_number": null,
"strength_unit": null
},
{
"name": "MAGNESIUM STEARATE",
"unii": "70097M6I30",
"strength_number": null,
"strength_unit": null
}
]
},
{
"number": 2,
"ndc_product_code": null,
"name": "Theramine",
"route": "ORAL",
"amount": "90",
"unit": "1",
"dosage_form": "CAPSULE",
"marketing_category": "MEDICAL FOOD",
"application_number": null,
"packaging_tree": [
{
"package_ndc_code": null,
"description": "90 in 1 BOTTLE",
"amount": "90",
"unit": "1",
"form": "BOTTLE"
}
],
"active_ingredients": [
{
"name": ".GAMMA.-AMINOBUTYRIC ACID",
"unii": "2ACZ6IPC6I",
"strength_number": "100",
"strength_unit": "mg/1"
}
],
"inactive_ingredients": [
{
"name": "MAGNESIUM STEARATE",
"unii": "70097M6I30",
"strength_number": null,
"strength_unit": null
},
{
"name": "CELLULOSE, MICROCRYSTALLINE",
"unii": "OP1R32D61U",
"strength_number": null,
"strength_unit": null
},
{
"name": "MALTODEXTRIN",
"unii": "7CVR7L4A2D",
"strength_number": null,
"strength_unit": null
},
{
"name": "GELATIN",
"unii": "2G86QN327L",
"strength_number": null,
"strength_unit": null
}
]
}
],
"packaging_tree": [
{
"package_ndc_code": "68405-0018-06",
"description": "1 in 1 KIT",
"amount": "1",
"unit": "1",
"form": "KIT"
}
]
}
A package that is found in kits will have a
part_ofarray to reference the kits. Some packages are only found in kits; those packages will have afor_partattribute in place of thefor_productattribute:
{
"package_ndc_code": "52959-0190-30",
"description": "60 in 1 BOTTLE",
"amount": "60",
"unit": "1",
"form": "BOTTLE",
"for_part": {
"ndc_product_code": "52959-0190",
"name": "NAPROXEN",
"active_ingredients": [
{
"name": "NAPROXEN",
"unii": "57Y76R9ATQ",
"strength_number": "250",
"strength_unit": "mg/1"
}
],
"inactive_ingredients": [
{
"name": "CROSCARMELLOSE SODIUM",
"unii": "M28OL1HH48",
"strength_number": null,
"strength_unit": null
},
{
"name": "POVIDONE",
"unii": "FZ989GH94E",
"strength_number": null,
"strength_unit": null
},
{
"name": "MAGNESIUM STEARATE",
"unii": "70097M6I30",
"strength_number": null,
"strength_unit": null
}
]
},
"packaging_tree": [
{
"package_ndc_code": "52959-0190-30",
"description": "60 in 1 BOTTLE",
"amount": "60",
"unit": "1",
"form": "BOTTLE"
}
],
"part_of": [
{
"package_ndc_code": "68405-0018-06",
"description": "1 in 1 KIT",
"product": {
"ndc_product_code": "68405-0018",
"name": "Theraproxen-90"
}
},
{
"package_ndc_code": "68405-0016-36",
"description": "1 in 1 KIT",
"product": {
"ndc_product_code": "68405-0016",
"name": "Trepoxen-250"
}
},
{
"package_ndc_code": "68405-0180-06",
"description": "1 in 1 KIT",
"product": {
"ndc_product_code": "68405-0180",
"name": "Theraproxen"
}
}
]
}
This endpoint retrieves a specific package based on Local Product ID.
HTTP Request
GET https://api.drugbank.com/v1/packages/<LOCAL_PRODUCT_ID>
URL Parameters
| Parameter | Description |
|---|---|
| LOCAL_PRODUCT_ID | The Local Product ID of the package to retrieve. |
Get the most current FDA label for a package
curl -L 'https://api.drugbank.com/v1/packages/50580-111-08/label'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"id": "5a42290a-810c-427c-a406-2df70a02c372",
"set_id": "81f3847f-99e8-4ff3-ad18-f8b8e1c54238",
"version": 1,
"current": true,
"title": "Tylenol Extra Strength - Acetaminophen LIQUID",
"product_type": "HUMAN OTC DRUG LABEL",
"effective_on": "2012-12-10",
"labeler_name": "McNeil Consumer Healthcare Div. McNeil-PPC, Inc",
"drugbank_status": "processed",
"first_product_marketing_started_on": "1998-04-01",
"last_product_marketing_ended_on": "2012-10-31",
"pmg_section_count": 6,
"hpi_section_count": 10,
"sli_section_count": 2,
"all_section_count": 17,
"abuse": null,
"accessories": null,
"active_ingredient": "<section id=\"section-2\" class=\"content-section\" data-section-code=\"55106-9\"><h1>Active ingredient (in each 15 mL = 1 tablespoon)</h1>\n<div class=\"conte...",
"adverse_reactions": null,
"alarms": null,
"animal_pharmacology_and_or_toxicology": null,
"ask_doctor": "<section id=\"section-5...",
"ask_doctor_or_pharmacist": "<section id=\"section-5...",
"assembly_or_installation_instructions": null,
"boxed_warning": null,
"calibration_instructions": null,
"carcinogenesis_and_mutagenesis_and_impairment_of_fertility": null,
"cleaning": null,
"clinical_pharmacology": null,
"clinical_studies": null,
"compatible_accessories": null,
"components": null,
"contraindications": null,
"controlled_substance": null,
"dependence": null,
"description": null,
"diagram_of_device": null,
"disposal_and_waste_handling": null,
"do_not_use": "<section id=\"section-5...",
"dosage_and_administration": "<section id=\"section-6\" class=\"content-section\" data-section-code=\"34068-7\"><h1>Directions</h1>\n<div class=\"content-section-inner\" id=\"\">\n<ul class=\"sq...",
"dosage_forms_and_strengths": null,
"drug_abuse_and_dependence": null,
"drug_interactions": null,
"drug_and_or_laboratory_test_interactions": null,
"environmental_warning": null,
"food_safety_warning": null,
"general_precautions": null,
"geriatric_use": null,
"guaranteed_analysis_of_feed": null,
"health_care_provider_letter": null,
"health_claim": null,
"how_supplied": null,
"inactive_ingredient": "<section id=\"section-8\" class=\"content-section\" data-section-code=\"51727-6\"><h1>Inactive ingredients</h1>\n<div class=\"content-section-inner\" id=\"\"><p>a...",
"indications_and_usage": "<section id=\"section-4\" class=\"content-section\" data-section-code=\"34067-9\"><h1>Uses</h1>\n<div class=\"content-section-inner\" id=\"\"><ul class=\"square\">\n...",
"information_for_owners_or_caregivers": null,
"information_for_patients": null,
"instructions_for_use": null,
"intended_use_of_the_device": null,
"keep_out_of_reach_of_children": "<section id=\"section-5...",
"labor_and_delivery": null,
"laboratory_tests": null,
"mechanism_of_action": null,
"microbiology": null,
"nonclinical_toxicology": null,
"nonteratogenic_effects": null,
"nursing_mothers": null,
"other_safety_information": null,
"overdosage": null,
"package_label_principal_display_panel": "<section id=\"section-10\" class=\"content-section\" data-section-code=\"51945-4\"><h1>PRINCIPAL DISPLAY PANEL</h1>\n<div class=\"content-section-inner\" id=\"\">...",
"patient_medication_information": null,
"pediatric_use": null,
"pharmacodynamics": null,
"pharmacogenomics": null,
"pharmacokinetics": null,
"precautions": null,
"pregnancy": null,
"pregnancy_or_breast_feeding": "<section id=\"section-5...",
"purpose": "<section id=\"section-3\" class=\"content-section\" data-section-code=\"55105-1\"><h1>Purpose</h1>\n<div class=\"content-section-inner\" id=\"\"><p>Pain reliever/...",
"questions": "<section id=\"section-9\" class=\"content-section\" data-section-code=\"53413-1\"><h1>Questions or comments?</h1>\n<div class=\"content-section-inner\" id=\"\"><p...",
"recent_major_changes": null,
"references": null,
"residue_warning": null,
"risks": null,
"route": null,
"safe_handling_warning": null,
"spl_indexing_data_elements": null,
"spl_medguide": null,
"spl_patient_package_insert": null,
"spl_product_data_elements": null,
"spl_unclassified": "<section id=\"section-1\" class=\"content-section\" data-section-code=\"42229-5\"><div class=\"content-section-inner\" id=\"\"><p><span class=\"bold italics\">Drug...",
"statement_of_identity": null,
"stop_use": "<section id=\"section-5...",
"storage_and_handling": "<section id=\"section-7\" class=\"content-section\" data-section-code=\"44425-7\"><h1>Other information</h1>\n<div class=\"content-section-inner\" id=\"\"><ul cla...",
"summary_of_safety_and_effectiveness": null,
"teratogenic_effects": null,
"troubleshooting": null,
"use_in_specific_populations": null,
"user_safety_warnings": null,
"warnings": "<section id=\"section-5\" class=\"content-section\" data-section-code=\"34071-1\"><h1>Warnings</h1>\n<div class=\"content-section-inner\" id=\"\">\n<section id=\"se...",
"warnings_and_cautions": null,
"when_using": null
}
This endpoint retrieves the most current FDA label for a package, based on NDC package ID.
HTTP Request
GET https://api.drugbank.com/v1/packages/<NDC_PACKAGE_ID>/label
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| content_format | html | Format to return for section content from label, either html (default) or text. |
Labels
Get a specific label
curl -L 'https://api.drugbank.com/v1/us/labels/77c79fcb-5b96-4a4f-8ce9-1603d39cb037'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"id": "77c79fcb-5b96-4a4f-8ce9-1603d39cb037",
"set_id": "51abd2ce-6be1-412d-b806-5f24935ac5e3",
"version": 17,
"current": false,
"title": "Zidovudine - Zidovudine SYRUP",
"product_type": "HUMAN PRESCRIPTION DRUG LABEL",
"effective_on": "2019-09-19",
"labeler_name": "Aurobindo Pharma Limited",
"drugbank_status": "archived",
"first_product_marketing_started_on": "2005-09-19",
"last_product_marketing_ended_on": null,
"pmg_section_count": 1,
"hpi_section_count": 19,
"sli_section_count": 8,
"all_section_count": 27,
"abuse": null,
"accessories": null,
"active_ingredient": null,
"adverse_reactions": "<section id=\"section-6\" class=\"content-section\" data-section-code=\"34084-4\"><h1>6 ADVERSE REACTIONS</h1>\n<div class=\"content-section-inner\" id=\"Section...",
"alarms": null,
"animal_pharmacology_and_or_toxicology": null,
"ask_doctor": null,
"ask_doctor_or_pharmacist": null,
"assembly_or_installation_instructions": null,
"boxed_warning": "<section id=\"boxed-warning\" data-section-code=\"34066-1\" class=\"content-section\"><h1>Boxed Warning</h1>\n<div class=\"boxed-warning-section\">\n<h2>WARNING:...",
"calibration_instructions": null,
"carcinogenesis_and_mutagenesis_and_impairment_of_fertility": "<section id=\"section-12...",
"cleaning": null,
"clinical_pharmacology": "<section id=\"section-11\" class=\"content-section\" data-section-code=\"34090-1\"><h1>12 CLINICAL PHARMACOLOGY</h1>\n<div class=\"content-section-inner\" id=\"S...",
"clinical_studies": "<section id=\"section-13\" class=\"content-section\" data-section-code=\"34092-7\"><h1>14 CLINICAL STUDIES</h1>\n<div class=\"content-section-inner\" id=\"Sectio...",
"compatible_accessories": null,
"components": null,
"contraindications": "<section id=\"section-4\" class=\"content-section\" data-section-code=\"34070-3\"><h1>4 CONTRAINDICATIONS</h1>\n<div class=\"content-section-inner\" id=\"Section...",
"controlled_substance": null,
"dependence": null,
"description": "<section id=\"section-10\" class=\"content-section\" data-section-code=\"34089-3\"><h1>11 DESCRIPTION</h1>\n<div class=\"content-section-inner\" id=\"Section_11\"...",
"diagram_of_device": null,
"disposal_and_waste_handling": null,
"do_not_use": null,
"dosage_and_administration": "<section id=\"section-2\" class=\"content-section\" data-section-code=\"34068-7\"><h1>2 DOSAGE AND ADMINISTRATION</h1>\n<div class=\"content-section-inner\" id=...",
"dosage_forms_and_strengths": "<section id=\"section-3\" class=\"content-section\" data-section-code=\"43678-2\"><h1>3 DOSAGE FORMS AND STRENGTHS</h1>\n<div class=\"content-section-inner\" id...",
"drug_abuse_and_dependence": null,
"drug_interactions": "<section id=\"section-7\" class=\"content-section\" data-section-code=\"34073-7\"><h1>7 DRUG INTERACTIONS</h1>\n<div class=\"content-section-inner\" id=\"Section...",
"drug_and_or_laboratory_test_interactions": null,
"environmental_warning": null,
"food_safety_warning": null,
"general_precautions": null,
"geriatric_use": "<section id=\"section-8...",
"guaranteed_analysis_of_feed": null,
"health_care_provider_letter": null,
"health_claim": null,
"how_supplied": "<section id=\"section-14\" class=\"content-section\" data-section-code=\"34069-5\"><h1>16 HOW SUPPLIED/STORAGE AND HANDLING</h1>\n<div class=\"content-section-...",
"inactive_ingredient": null,
"indications_and_usage": "<section id=\"section-1\" class=\"content-section\" data-section-code=\"34067-9\"><h1>1 INDICATIONS AND USAGE</h1>\n<div class=\"content-section-inner\" id=\"Sec...",
"information_for_owners_or_caregivers": null,
"information_for_patients": "<section id=\"section-15\" class=\"content-section\" data-section-code=\"34076-0\"><h1>17 PATIENT COUNSELING INFORMATION</h1>\n<div class=\"content-section-inn...",
"instructions_for_use": null,
"intended_use_of_the_device": null,
"keep_out_of_reach_of_children": null,
"labor_and_delivery": "<section id=\"section-8...",
"laboratory_tests": null,
"mechanism_of_action": "<section id=\"section-11...",
"microbiology": null,
"nonclinical_toxicology": "<section id=\"section-12\" class=\"content-section\" data-section-code=\"43680-8\"><h1>13 NONCLINICAL TOXICOLOGY</h1>\n<div class=\"content-section-inner\" id=\"...",
"nonteratogenic_effects": null,
"nursing_mothers": null,
"other_safety_information": null,
"overdosage": "<section id=\"section-9\" class=\"content-section\" data-section-code=\"34088-5\"><h1>10 OVERDOSAGE</h1>\n<div class=\"content-section-inner\" id=\"Section_10\">\n...",
"package_label_principal_display_panel": "<section id=\"section-16\" class=\"content-section\" data-section-code=\"51945-4\"><h1>PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg/mL (240 mL Bottle)</h1>\n...",
"patient_medication_information": null,
"pediatric_use": "<section id=\"section-8...",
"pharmacodynamics": null,
"pharmacogenomics": null,
"pharmacokinetics": "<section id=\"section-11...",
"precautions": null,
"pregnancy": "<section id=\"section-8...",
"pregnancy_or_breast_feeding": null,
"purpose": null,
"questions": null,
"recent_major_changes": null,
"references": null,
"residue_warning": null,
"risks": null,
"route": null,
"safe_handling_warning": null,
"spl_indexing_data_elements": null,
"spl_medguide": null,
"spl_patient_package_insert": null,
"spl_product_data_elements": null,
"spl_unclassified": "<section id=\"section-1...",
"statement_of_identity": null,
"stop_use": null,
"storage_and_handling": null,
"summary_of_safety_and_effectiveness": null,
"teratogenic_effects": null,
"troubleshooting": null,
"use_in_specific_populations": "<section id=\"section-8\" class=\"content-section\" data-section-code=\"43684-0\"><h1>8 USE IN SPECIFIC POPULATIONS</h1>\n<div class=\"content-section-inner\" i...",
"user_safety_warnings": null,
"warnings": null,
"warnings_and_cautions": "<section id=\"section-5\" class=\"content-section\" data-section-code=\"43685-7\"><h1>5 WARNINGS AND PRECAUTIONS</h1>\n<div class=\"content-section-inner\" id=\"...",
"when_using": null
}
This endpoint retrieves a specific FDA label (SPL) based on SPL document ID or the SPL set ID.
HTTP Request
GET https://api.drugbank.com/v1/us/labels/<DOCUMENT_ID>
URL Parameters
| Parameter | Description |
|---|---|
| ID | The SPL document ID or set ID of the label to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| content_format | html | Format to return for section content from label, either html (default) or text. |
Label sections
The label sections are returned by default as html.
The sections contain structured information such as headings, tables, and images.
However, you can optionally return the content as text using the content_format parameter.
The html that is returned contains a wrapping <section> tag, with a unique ID that
is used to link to that section within the other sections.
Images within the sections are linked to DrugBank assets. The tags within sections are easily styled, and you can generate a styled label. See the example label here.
The content of a label is broken down into unique, categorized sections (listed in the
tables below). Different sections are relevant for each of the labels modules: hpi (Health
Profesional Information), pmg (Patient Medication Guides), or sli (Supplementary Label
Information). Total section counts for each module as well as the entire label can be found
in the API response (note that the same section can appear in more than one module).
Abuse and overdosage
| Section | Description |
|---|---|
| drug_abuse_and_dependence | Information about whether the drug is a controlled substance, the types of abuse that can occur with the drug, and adverse reactions pertinent to those types of abuse. |
| abuse | Information about the types of abuse that can occur with the drug and adverse reactions pertinent to those types of abuse, primarily based on human data. May include descriptions of particularly susceptible patient populations. |
| controlled_substance | Information about the schedule in which the drug is controlled by the Drug Enforcement Administration, if applicable. |
| dependence | Information about characteristic effects resulting from both psychological and physical dependence that occur with the drug, the quantity of drug over a period of time that may lead to tolerance or dependence, details of adverse effects related to chronic abuse and the effects of abrupt withdrawl, procedures necessary to diagnose the dependent state, and principles of treating the effects of abrupt withdrawal. |
| overdosage | Information about signs, symptoms, and laboratory findings of acute ovedosage and the general principles of overdose treatment. |
Adverse effects and interactions
| Section | Description |
|---|---|
| adverse_reactions | Information about undesirable effects, reasonably associated with use of the drug, that may occur as part of the pharmacological action of the drug or may be unpredictable in its occurrence. Adverse reactions include those that occur with the drug, and if applicable, with drugs in the same pharmacologically active and chemically related class. There is considerable variation in the listing of adverse reactions. They may be categorized by organ system, by severity of reaction, by frequency, by toxicological mechanism, or by a combination of these. |
| drug_interactions | Information about and practical guidance on preventing clinically significant drug/drug and drug/food interactions that may occur in people taking the drug. |
| drug_and_or_laboratory_test_ interactions |
Information about any known interference by the drug with laboratory tests. |
Clinical pharmacology
| Section | Description |
|---|---|
| clinical_pharmacology | Information about the clinical pharmacology and actions of the drug in humans. |
| mechanism_of_action | Information about the established mechanism(s) of the drug’s action in humans at various levels (for example receptor, membrane, tissue, organ, whole body). If the mechanism of action is not known, this field contains a statement about the lack of information. |
| pharmacodynamics | Information about any biochemical or physiologic pharmacologic effects of the drug or active metabolites related to the drug’s clinical effect in preventing, diagnosing, mitigating, curing, or treating disease, or those related to adverse effects or toxicity. |
| pharmacokinetics | Information about the clinically significant pharmacokinetics of a drug or active metabolites, for instance pertinent absorption, distribution, metabolism, and excretion parameters. |
Indications, usage, and dosage
| Section | Description |
|---|---|
| indications_and_usage | A statement of each of the drug product’s indications for use, such as for the treatment, prevention, mitigation, cure, or diagnosis of a disease or condition, or of a manifestation of a recognized disease or condition, or for the relief of symptoms associated with a recognized disease or condition. This field may also describe any relevant limitations of use. |
| contraindications | Information about situations in which the drug product is contraindicated or should not be used because the risk of use clearly outweighs any possible benefit, including the type and nature of reactions that have been reported. |
| dosage_and_administration | Information about the drug product’s dosage and administration recommendations, including starting dose, dose range, titration regimens, and any other clinically sigificant information that affects dosing recommendations. |
| dosage_forms_and_strengths | Information about all available dosage forms and strengths for the drug product to which the labeling applies. This field may contain descriptions of product appearance. |
| purpose | Information about the drug product’s indications for use. |
| description | General information about the drug product, including the proprietary and established name of the drug, the type of dosage form and route of administration to which the label applies, qualitative and quantitative ingredient information, the pharmacologic or therapeutic class of the drug, and the chemical name and structural formula of the drug. |
| active_ingredient | A list of the active, medicinal ingredients in the drug product. |
| inactive_ingredient | A list of inactive, non-medicinal ingredients in a drug product. |
| spl_product_data_elements | Typically a list of ingredients in a drug product. |
Patient information
| Section | Description |
|---|---|
| spl_patient_package_insert | Information necessary for patients to use the drug safely and effectively. |
| information_for_patients | Information necessary for patients to use the drug safely and effectively, such as precautions concerning driving or the concomitant use of other substances that may have harmful additive effects. |
| information_for_owners_or_caregivers | Information to be supplied to a guardian/caregiver overseeing the use of the drug product. |
| instructions_for_use | Information about safe handling and use of the drug product. |
| ask_doctor | Information about when a doctor should be consulted about existing conditions or sumptoms before using the drug product, including all warnings for persons with certain preexisting conditions (excluding pregnancy) and all warnings for persons experiencing certain symptoms. The warnings under this heading are those intended only for situations in which consumers should not use the product until a doctor is consulted. |
| ask_doctor_or_pharmacist | Information about when a doctor or pharmacist should be consulted about drug/drug or drug/food interactions before using a drug product. |
| do_not_use | Information about all contraindications for use. These contraindications are absolute and are intended for situations in which consumers should not use the product unless a prior diagnosis has been established by a doctor or for situations in which certain consumers should not use the product under any circumstances regardless of whether a doctor or health professional is consulted. |
| keep_out_of_reach_of_children | Information pertaining to whether the product should be kept out of the reach of children, and instructions about what to do in the case of accidental contact or ingestion, if appropriate. |
| other_safety_information | Information about safe use and handling of the product that may not have been specified in another field. |
| questions | A telephone number of a source to answer questions about a drug product. Sometimes available days and times are also noted. |
| stop_use | Information about when use of the drug product should be discontinued immediately and a doctor consulted. Includes information about any signs of toxicity or other reactions that would necessitate immediately discontinuing use of the product. |
| when_using | Information about side effects that people may experience, and the substances (e.g. alcohol) or activities (e.g. operating machinery, driving a car) to avoid while using the drug product. |
| patient_medication_information | Information or instructions to patients about safe use of the drug product, sometimes including a reference to a patient medication guide or counseling materials. |
| spl_medguide | Information about the patient medication guide that accompanies the drug product. Certain drugs must be dispensed with an accompanying medication guide. This field may contain information about when to consult the medication guide and the contents of the medication guide. |
Special populations
| Section | Description |
|---|---|
| use_in_specific_populations | Information about use of the drug by patients in specific populations, including pregnant women and nursing mothers, pediatric patients, and geriatric patients. |
| pregnancy | Information about effects the drug may have on pregnant women or on a fetus. This field may be ommitted if the drug is not absorbed systemically and the drug is not known to have a potential for indirect harm to the fetus. It may contain information about the established pregnancy category classification for the drug (that information is nominally listed in the teratogenic_effects field, but may be listed here instead). |
| teratogenic_effects | Pregnancy category A: Adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in the first trimester of pregnancy, and there is no evidence of a risk in later trimesters. Pregnancy category B: Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women. Pregnancy category C: Animal reproduction studies have shown an adverse effect on the fetus, there are no adequate and well-controlled studies in humans, and the benefits from the use of the drug in pregnant women may be acceptable despite its potential risks. Pregnancy category D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but the potential benefits from the use of the drug in pregnant women may be acceptable despite its potential risks (for example, if the drug is needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective). Pregnancy category X: Studies in animals or humans have demonstrated fetal abnormalities or there is positive evidence of fetal risk based on adverse reaction reports from investigational or marketing experience, or both, and the risk of the use of the drug in a pregnant woman clearly outweighs any possible benefit (for example, safer drugs or other forms of therapy are available). |
| labor_and_delivery | Information about the drug’s use during labor or delivery, whether or not the use is stated in the indications section of the labeling, including the effect of the drug on the mother and fetus, on the duration of labor or delivery, on the possibility of delivery-related interventions, and the effect of the drug on the later growth, development, and functional maturation of the child. |
| nursing_mothers | Information about excretion of the drug in human milk and effects on the nursing infant, including pertinent adverse effects observed in animal offspring. |
| pregnancy_or_breast_feeding | Details about usage of the product while pregnant or breast feeding. |
| pediatric_use | Information about any limitations on any pediatric indications, needs for specific monitoring, hazards associated with use of the drug in any subsets of the pediatric population (such as neonates, infants, children, or adolescents), differences between pediatric and adult responses to the drug, and other information related to the safe and effective pediatric use of the drug. |
| geriatric_use | Information about any limitations on any geriatric indications, needs for specific monitoring, hazards associated with use of the drug in the geriatric population. |
Nonclinical toxicology
| Section | Description |
|---|---|
| nonclinical_toxicology | Information about toxicology in non-human subjects. |
| nonteratogenic_effects | Details about potential neonatal complications when used by a pregnant mother. |
| carcinogenesis_and_mutagenesis_ and_impairment_of_fertility |
Information about carcinogenic, mutagenic, or fertility impairment potential revealed by studies in animals. Information from human data about such potential is part of the warnings field. |
| animal_pharmacology_and_ or_toxicology |
Information from studies of the drug in animals, if the data were not relevant to nor included in other parts of the labeling. Most labels do not contain this field. |
References
| Section | Description |
|---|---|
| clinical_studies | This field may contain references to clinical studies in place of detailed discussion in other sections of the labeling. |
| references | May contain references when prescription drug labeling must summarize or otherwise relay on a recommendation by an authoritative scientific body, or on a standardized methodology, scale, or technique, because the information is important to prescribing decisions. |
Supply, storage, and handling
| Section | Description |
|---|---|
| how_supplied | Information about the available dosage forms to which the labeling applies, and for which the manufacturer or distributor is responsible. This field ordinarily includes the strength of the dosage form (in metric units), the units in which the dosage form is available for prescribing, appropriate information to facilitate identification of the dosage forms (such as shape, color, coating, scoring, and National Drug Code), and special handling and storage condition information. |
| storage_and_handling | Information about safe storage and handling of the drug product. |
| safe_handling_warning | Warning about how to safely handle the drug or device. |
Warnings and precautions
| Section | Description |
|---|---|
| boxed_warning | Information about contraindications or serious warnings, particularly those that may lead to death or serious injury (also known as “blackbox warnings”). |
| user_safety_warnings | When a drug can pose a hazard to human health by contact, inhalation, ingestion, injection, or by any exposure, this field contains information which can prevent or decrease the possibility of harm. |
| warnings | Information about serious adverse reactions and potential safety hazards, including limitations in use imposed by those hazards and steps that should be taken if they occur. |
| warnings_and_cautions | Warnings and cautions to take while using the product. |
| general_precautions | Information about any special care to be exercised for safe and effective use of the drug. |
Other fields
In addition, there are several sections that are less well defined and are less commonly used.
| Section | Description |
|---|---|
| accessories | Details about accessories that can be used with the product. |
| alarms | Details regarding major adverse reactions. Typically not present. |
| assembly_or_installation_instructions | Instructions on assembling/installing a drug product (typically applies to kits). |
| calibration_instructions | Instructions on calibrating a device. |
| cleaning | Details on how to clean a device. |
| compatible_accessories | Details about relevant compatible accessories/parts/products. |
| components | Details of the various components in a product. |
| diagram_of_device | A picture of the delivery device for a drug product. |
| disposal_and_waste_handling | Details about how to dispose of an expired or used drug product. |
| environmental_warning | Details about environmental risks a product may pose. |
| food_safety_warning | Warning about the effects of foods on the drug, and vice versa. |
| guaranteed_analysis_of_feed | A guarantee about the content of a single serving of a product (for animal drug labels). |
| health_care_provider_letter | A letter, from the manufacturer, intended for the health care provider (typically regarding shortages, etc.). |
| health_claim | A specific health claim being made about the drug product. |
| intended_use_of_the_device | Details about how the device is intended to be used. |
| laboratory_tests | Information on laboratory tests helpful in following the patient’s response to the drug or in identifying possible adverse reactions. If appropriate, information may be provided on such factors as the range of normal and abnormal values expected in the particular situation and the recommended frequency with which tests should be performed before, during, and after therapy. |
| microbiology | Details about microbiology, usually present for anti-bacterials. |
| package_label_principal_display_panel | The content of the principal display panel of the product package, usually including the product’s name, dosage forms, and other key information about the drug product. |
| pharmacogenomics | A description of the pharmacogenomics for a drug. |
| precautions | Information about any special care to be exercised for safe and effective use of the drug. |
| recent_major_changes | A list of the section(s) that contain substantive changes that have been approved by FDA in the product labeling. The headings and subheadings, if appropriate, affected by the change are listed together with each section’s identifying number and the month and year on which the change was incorporated in the labeling. |
| residue_warning | Warnings about residues present on the product. |
| risks | Details about risks when usig the product. |
| route | Details about the route of administration for a product. |
| statement_of_identity | Details about the content of a nutritional supplement. |
| summary_of_safety_and_effectiveness | Summarization of safety and effectiveness. |
| troubleshooting | Troubleshooting guide for patient instructions. |
| spl_indexing_data_elements | Used for indexing a label. |
| spl_unclassified | Information not classified as belonging to one of the other fields. Approximately 40% of labeling with effective_time between June 2009 and August 2014 have information in this field. |
Get the rendered contents of a specific FDA label
curl -L 'https://api.drugbank.com/v1/us/labels/77c79fcb-5b96-4a4f-8ce9-1603d39cb037/rendered?content=full'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"id": "77c79fcb-5b96-4a4f-8ce9-1603d39cb037",
"set_id": "51abd2ce-6be1-412d-b806-5f24935ac5e3",
"version": 17,
"current": false,
"title": "Zidovudine - Zidovudine SYRUP",
"product_type": "HUMAN PRESCRIPTION DRUG LABEL",
"effective_on": "2019-09-19",
"labeler_name": "Aurobindo Pharma Limited",
"drugbank_status": "archived",
"first_product_marketing_started_on": "2005-09-19",
"last_product_marketing_ended_on": null,
"pmg_section_count": 1,
"hpi_section_count": 19,
"sli_section_count": 8,
"all_section_count": 27,
"content": "<div class=\"container-fluid\"><h1 class=\"display-1\">Zidovudine - Zidovudine SYRUP</h1><div class=\"row\" id=\"drugbank-label\"><div class=\"col\" id=\"table-of..."
}
Including label.css and rendered content in HTML:
<html>
<head>
<meta charset="UTF-8">
<link rel="stylesheet" href="label.css" />
</head>
<body>
<!--label content-->
</body>
</html>
This endpoint retrieves a specific FDA label (SPL) based on SPL document ID
or the SPL set ID. It returns the applicable sections of a label grouped in
one HTML content field.
Images within the sections are linked to DrugBank assets. The tags within sections are easily styled, and you can generate a styled label. See the example label here.
You can style this label using your own CSS. You can download the styles used in the example above here. They will need to be linked in the HTML where you display the label.
Note that the content parameter is required and API responses will be filtered accordingly - full, hpi, pmg, or sli.
HTTP Request
GET https://api.drugbank.com/v1/us/labels/<DOCUMENT_ID>/rendered
URL Parameters
| Parameter | Description |
|---|---|
| ID | The SPL document ID or set ID of the label to retrieve. |
Query Parameters
| Parameter | Default | Description |
|---|---|---|
| content | The group of sections to include in the rendered label that must be set to one of the following. full renders the entire label. hpi (Health Profesional Information), pmg (Patient Medication Guides), or sli (Supplementary Label Information) will render smaller versions of the label, serving specific purposes. |
Get historic documents for a specific FDA label
curl -L 'https://api.drugbank.com/v1/us/labels/77c79fcb-5b96-4a4f-8ce9-1603d39cb037/set'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"id": "cc3a8968-9b99-468c-99e6-4f1161e84b2a",
"version": 21,
"current": true,
"title": "Zidovudine - Zidovudine SYRUP",
"product_type": "HUMAN PRESCRIPTION DRUG LABEL",
"labeler_name": "Aurobindo Pharma Limited",
"drugbank_status": "processed"
},
{
"id": "b386d167-ddd7-4163-b965-19a3bfaf01d4",
"version": 20,
"current": false,
"title": "Zidovudine - Zidovudine SYRUP",
"product_type": "HUMAN PRESCRIPTION DRUG LABEL",
"labeler_name": "Aurobindo Pharma Limited",
"drugbank_status": "archived"
},
"..."
]
This endpoint retrieves other labels with the same set ID for a specific FDA label (SPL) based on SPL document ID. A set ID is a GUID (globally unique identifier) for the document that remains constant through all versions/revisions of the document.
HTTP Request
GET https://api.drugbank.com/v1/us/labels/<DOCUMENT_ID>/set
URL Parameters
| Parameter | Description |
|---|---|
| ID | The SPL document ID or set ID of the label to retrieve. |
References
An example of the structure of a reference list:
{
"references": {
"literature_references": [
{
"ref_id": "A463",
"pubmed_id": 15879007,
"citation": "Kis B, Snipes JA, Busija DW: Acetaminophen and the cyclooxygenase-3 puzzle: sorting out facts, fictions, and uncertainties. J Pharmacol Exp Ther. 2005 Oct;315(1):1-7. Epub 2005 May 6."
},
{
"ref_id": "A464",
"pubmed_id": 16413237,
"citation": "Aronoff DM, Oates JA, Boutaud O: New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 2006 Jan;79(1):9-19."
}
],
"textbooks": [
{
"ref_id": "T518",
"isbn": null,
"citation": "Valerie Gerriets; Thomas M. Nappe (2019). Acetaminophen. StatPearls publishing."
}
],
"external_links": [
{
"ref_id": "L5756",
"title": "Acetaminophen tablet, DailyMed",
"url": "https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c3b408ff-f47b-4fee-ade5-5db6e25f4ee0"
},
{
"ref_id": "L5774",
"title": "Acetaminophen effervescent tablets, Cleveland Clinic",
"url": "https://my.clevelandclinic.org/health/drugs/18282-acetaminophen-effervescent-tablets"
}
],
"attachments": [
{
"ref_id": "F4124",
"title": "Acetaminophen monograph, suppository",
"url": "//s3-us-west-2.amazonaws.com/drugbank-qa/cite_this/attachments/files/000/004/124/original/Acetaminophen_monograph__suppository.pdf?1553636652"
}
]
}
}
Certain fields, such as drug descriptions and drug-drug interaction descriptions, include citations within the text. These citations are in the format:
[<letter><number>]
Where the letter indicates the source type of the citation and the number is the identifier (e.g. [A001]). The citation source types are listed below:
| Letter | Type |
|---|---|
| A | Literature article |
| T | Textbook |
| L | External link |
| F | File attachment |
The corresponding citation details can be returned within the JSON object by including include_references=true as a parameter in the URL.
API Versions
Accessing API versions
| Version | Released | Removed |
|---|---|---|
| v0 | July, 2016 | March, 2022 |
| v1 | September, 2016 | Current |
You can access the latest version of the API docs using the base docs url:
This will always direct you to the newest version of the API.
API Upgrades and Changes
Keeping track of changes and upgrades to the DrugBank API
Your API version (e.g. v1) controls the API behavior you see (e.g., what properties you see in responses, what parameters you’re permitted to send in requests, etc.). When we change the API in a backwards-incompatible way, we release a new version, but to avoid breaking your code, we maintain older versions for a reasonable period of time and support users in their migration. Older versions will not be removed without ensuring full migration for all customers, however we encourage customers to keep up to date with newer versions to take advantage of improvements and additions.
Backwards-compatible changes
DrugBank considers the following changes to be backwards-compatible:
- Adding new API resources.
- Adding new optional request parameters to existing API methods.
- Adding new properties to existing API responses.
- Changing the order of properties in existing API responses.
- Changing the length or format of opaque strings, such as error messages and other human-readable strings.
- Improving the relevance or ordering of search results
You can safely assume object IDs will not change in length, and will never exceed 255 characters.
Upgrading your API version
If you’re running an older version of the API, upgrade to the latest version to take advantage of new functionality or to streamline responses so the API is faster for you. Upgrading your API version can affect:
- The parameters you can send and the structure of objects returned.
- The parameters in headers that are required and returned.
- How request tallies are calculated for billing purposes.
- How authentication is performed.
- Whether endpoints are available. Endpoints may be deprecated with major version changes.
To upgrade to the latest version, change the version number in requests sent to the API (e.g. change “v0” to “v1”). You can also use two versions of the API at the same time to upgrade iteratively.
When performing an API upgrade, make sure that you review the change log below, and that your tests pass against the endpoints you are using in your application. You can run your tests against the new version while still using your previous version in production.
Clinical API changelog
The changelog is a list of updates in the clinical API. Minor backwards-compatible additions and improvements (e.g. increasing the performance of an endpoint) may not appear in this list.
October 12, 2022
- New Added a new Allergies section.
- New Added an endpoint to view allergy alternatives for a drug based on therapeutic categories and cross-sensitivities.
- New Added endpoints to view drug alternatives for a product allergy and a product concept allergy based on therapeutic categories and cross-sensitivities.
- New Added a drug-allergy checker endpoint.
- New Added allergy presentation search and get a presentation endpoints.
- New Added an allergy presentation checker endpoint.
September 15, 2022
- New Therapeutic alternatives are now constrained to clinically-relevant therapeutic categories for more targeted results.
August 29, 2022
- New Restricted the therapeutic categories returned for individual drugs throughout the API to those for which the drug has a therapeutic categorization.
August 24, 2022
- New Added validation for the number of input codes to our DDI endpoint.
August 04, 2022
- New Added condition and ICD-10 search plugins as “plug and play” options for our search conditions and ICD-10 search endpoints, respectively.
June 24, 2022
- New Added the drug-drug interactions plugin as a “plug and play” option for our DDI endpoints.
June 15, 2022
curl -L 'https://api.drugbank.com/v1/conditions?q=arth'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"hits": [
{
"field": "general title",
"value": "<em>Arth</em>ritis/<em>Arth</em>rosis"
},
{
"field": "title",
"value": "<em>Arth</em>ritis/<em>Arth</em>rosis"
}
],
"name": "Arthritis/Arthrosis",
"drugbank_id": "DBCOND0086825",
"uniprot_id": null,
"meddra_id": null,
"icd10_id": null,
"snomed_id": "d/400379010"
},
{
"hits": [
{
"field": "general title",
"value": "<em>Arth</em>ritis and <em>Arth</em>ralgia"
},
{
"field": "title",
"value": "<em>Arth</em>ritis and <em>Arth</em>ralgia"
}
],
"name": "Arthritis and Arthralgia",
"drugbank_id": "DBCOND0142150",
"uniprot_id": null,
"meddra_id": null,
"icd10_id": null,
"snomed_id": null
},
"..."
]
- New Updated conditions search to facilitate autocomplete and fuzzy searching.
June 01, 2022
Search ICD-10 concepts by code or description.
curl -L 'https://api.drugbank.com/v1/icd10?q=alz'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"hits": [
{
"field": "description",
"value": "<em>Alz</em>heimer's disease"
}
],
"identifier": "G30",
"description": "Alzheimer's disease"
}
]
- New Added an ICD-10 Search endpoint.
May 16, 2022
- New Therapeutic alternatives are now constrained to drugs that specifically have therapeutic categorizations.
April 30, 2022
- Removed “Find DDI with Product Names” is no longer supported.
April 13, 2022
- New Added stricter but more informative validation of
per_pageparameter.
April 06, 2022
- New Added an
include_referencesquery parameter when retrieving adverse effects for drugs, products, and product concepts.
March 31, 2022
- Removed v0 of the API is no longer supported.
March 23, 2022
- New Added the medication search plugin as a “plug and play” option for our medication name search endpoint.
March 09, 2022
- New Added drug products that have received emergency use authorization across all regions.
February 09, 2022
Partial drug alternatives for DB01060 (Amoxicillin). Full result also contains the Anti-Bacterial Agents and Penicillins categories.
[
{
"drugbank_id": "DBCAT003682",
"name": "Aminopenicillins",
"mesh_id": null,
"mesh_tree_numbers": [],
"atc_code": null,
"atc_level": null,
"categorization_kind": "therapeutic",
"synonyms": [],
"description": null,
"drugs": [
{
"drugbank_id": "DB00415",
"name": "Ampicillin"
}
]
}
]
- New Added an endpoint to view therapeutic alternatives for a drug based on therapeutic categories.
- New Added endpoints to view drug alternatives for a product and a product concept based on therapeutic categories.
February 03, 2022
New section count metadata
{
"pmg_section_count": 1,
"hpi_section_count": 18,
"sli_section_count": 8,
"all_section_count": 27
}
- New Added total section counts for each drug label module (
pmg,hpi,sli) as well as the entire label.
October 15, 2021
Get a specific Singapore product
curl -L 'https://api.drugbank.com/v1/products/SIN11734P?region=sg'
-H 'Authorization: myapikey'
- New Added support for new region:
- Singapore
September 15, 2021
curl -L -X POST 'https://api.drugbank.com/v1/tokens' \
-H 'Content-Type: application/json' \
-H 'Authorization: myapikey' \
-H 'Cache-Control: no-cache' -d '{
"ttl": "15m"
}'
Example command returns JSON structured like this (results may be abbreviated):
{
"token": "eyJhbGciOiJIUzI1NiJ9.eyJrZXlfaWQiOjk5MiwiZXhwIjoxNzE4MDQxNzg1fQ.4K2UNBPlxppfFsO7NHC9hF3qY9LZRq512EYzpiRUDlQ"
}
- New Added the ability to define the unit of a
ttlas minutes ('m') or hours ('h'). If no unit is specified, attlof hours is assumed. See token authentication for more details.
June 21, 2021
curl -L 'https://api.drugbank.com/v1/ingredient_names?q=acetaminophen'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
[
{
"hits": [
{
"field": "name.autocomplete",
"value": "<em>Acetaminophen</em>"
},
{
"field": "synonyms",
"value": "<em>Acetaminophen</em>"
}
],
"drugbank_id": "DB00316",
"name": "Acetaminophen",
"cas_number": "103-90-2"
}
]
- New Added an ingredient name search endpoint for searching drug ingredients only, in addition to the existing drug name and prescribable name searches.
March 29, 2021
curl -L 'https://api.drugbank.com/v1/us/labels/77c79fcb-5b96-4a4f-8ce9-1603d39cb037'
-H 'Authorization: myapikey'
Example command returns JSON structured like this (results may be abbreviated):
{
"id": "77c79fcb-5b96-4a4f-8ce9-1603d39cb037",
"set_id": "51abd2ce-6be1-412d-b806-5f24935ac5e3",
"version": 17,
"current": false,
"title": "Zidovudine - Zidovudine SYRUP",
"product_type": "HUMAN PRESCRIPTION DRUG LABEL",
"effective_on": "2019-09-19",
"labeler_name": "Aurobindo Pharma Limited",
"drugbank_status": "archived",
"first_product_marketing_started_on": "2005-09-19",
"last_product_marketing_ended_on": null,
"pmg_section_count": 1,
"hpi_section_count": 19,
"sli_section_count": 8,
"all_section_count": 27,
"abuse": null,
"accessories": null,
"active_ingredient": null,
"adverse_reactions": "<section id=\"section-6\" class=\"content-section\" data-section-code=\"34084-4\"><h1>6 ADVERSE REACTIONS</h1>\n<div class=\"content-section-inner\" id=\"Section...",
"alarms": null,
"animal_pharmacology_and_or_toxicology": null,
"ask_doctor": null,
"ask_doctor_or_pharmacist": null,
"assembly_or_installation_instructions": null,
"boxed_warning": "<section id=\"boxed-warning\" data-section-code=\"34066-1\" class=\"content-section\"><h1>Boxed Warning</h1>\n<div class=\"boxed-warning-section\">\n<h2>WARNING:...",
"calibration_instructions": null,
"carcinogenesis_and_mutagenesis_and_impairment_of_fertility": "<section id=\"section-12...",
"cleaning": null,
"clinical_pharmacology": "<section id=\"section-11\" class=\"content-section\" data-section-code=\"34090-1\"><h1>12 CLINICAL PHARMACOLOGY</h1>\n<div class=\"content-section-inner\" id=\"S...",
"clinical_studies": "<section id=\"section-13\" class=\"content-section\" data-section-code=\"34092-7\"><h1>14 CLINICAL STUDIES</h1>\n<div class=\"content-section-inner\" id=\"Sectio...",
"compatible_accessories": null,
"components": null,
"contraindications": "<section id=\"section-4\" class=\"content-section\" data-section-code=\"34070-3\"><h1>4 CONTRAINDICATIONS</h1>\n<div class=\"content-section-inner\" id=\"Section...",
"controlled_substance": null,
"dependence": null,
"description": "<section id=\"section-10\" class=\"content-section\" data-section-code=\"34089-3\"><h1>11 DESCRIPTION</h1>\n<div class=\"content-section-inner\" id=\"Section_11\"...",
"diagram_of_device": null,
"disposal_and_waste_handling": null,
"do_not_use": null,
"dosage_and_administration": "<section id=\"section-2\" class=\"content-section\" data-section-code=\"34068-7\"><h1>2 DOSAGE AND ADMINISTRATION</h1>\n<div class=\"content-section-inner\" id=...",
"dosage_forms_and_strengths": "<section id=\"section-3\" class=\"content-section\" data-section-code=\"43678-2\"><h1>3 DOSAGE FORMS AND STRENGTHS</h1>\n<div class=\"content-section-inner\" id...",
"drug_abuse_and_dependence": null,
"drug_interactions": "<section id=\"section-7\" class=\"content-section\" data-section-code=\"34073-7\"><h1>7 DRUG INTERACTIONS</h1>\n<div class=\"content-section-inner\" id=\"Section...",
"drug_and_or_laboratory_test_interactions": null,
"environmental_warning": null,
"food_safety_warning": null,
"general_precautions": null,
"geriatric_use": "<section id=\"section-8...",
"guaranteed_analysis_of_feed": null,
"health_care_provider_letter": null,
"health_claim": null,
"how_supplied": "<section id=\"section-14\" class=\"content-section\" data-section-code=\"34069-5\"><h1>16 HOW SUPPLIED/STORAGE AND HANDLING</h1>\n<div class=\"content-section-...",
"inactive_ingredient": null,
"indications_and_usage": "<section id=\"section-1\" class=\"content-section\" data-section-code=\"34067-9\"><h1>1 INDICATIONS AND USAGE</h1>\n<div class=\"content-section-inner\" id=\"Sec...",
"information_for_owners_or_caregivers": null,
"information_for_patients": "<section id=\"section-15\" class=\"content-section\" data-section-code=\"34076-0\"><h1>17 PATIENT COUNSELING INFORMATION</h1>\n<div class=\"content-section-inn...",
"instructions_for_use": null,
"intended_use_of_the_device": null,
"keep_out_of_reach_of_children": null,
"labor_and_delivery": "<section id=\"section-8...",
"laboratory_tests": null,
"mechanism_of_action": "<section id=\"section-11...",
"microbiology": null,
"nonclinical_toxicology": "<section id=\"section-12\" class=\"content-section\" data-section-code=\"43680-8\"><h1>13 NONCLINICAL TOXICOLOGY</h1>\n<div class=\"content-section-inner\" id=\"...",
"nonteratogenic_effects": null,
"nursing_mothers": null,
"other_safety_information": null,
"overdosage": "<section id=\"section-9\" class=\"content-section\" data-section-code=\"34088-5\"><h1>10 OVERDOSAGE</h1>\n<div class=\"content-section-inner\" id=\"Section_10\">\n...",
"package_label_principal_display_panel": "<section id=\"section-16\" class=\"content-section\" data-section-code=\"51945-4\"><h1>PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg/mL (240 mL Bottle)</h1>\n...",
"patient_medication_information": null,
"pediatric_use": "<section id=\"section-8...",
"pharmacodynamics": null,
"pharmacogenomics": null,
"pharmacokinetics": "<section id=\"section-11...",
"precautions": null,
"pregnancy": "<section id=\"section-8...",
"pregnancy_or_breast_feeding": null,
"purpose": null,
"questions": null,
"recent_major_changes": null,
"references": null,
"residue_warning": null,
"risks": null,
"route": null,
"safe_handling_warning": null,
"spl_indexing_data_elements": null,
"spl_medguide": null,
"spl_patient_package_insert": null,
"spl_product_data_elements": null,
"spl_unclassified": "<section id=\"section-1...",
"statement_of_identity": null,
"stop_use": null,
"storage_and_handling": null,
"summary_of_safety_and_effectiveness": null,
"teratogenic_effects": null,
"troubleshooting": null,
"use_in_specific_populations": "<section id=\"section-8\" class=\"content-section\" data-section-code=\"43684-0\"><h1>8 USE IN SPECIFIC POPULATIONS</h1>\n<div class=\"content-section-inner\" i...",
"user_safety_warnings": null,
"warnings": null,
"warnings_and_cautions": "<section id=\"section-5\" class=\"content-section\" data-section-code=\"43685-7\"><h1>5 WARNINGS AND PRECAUTIONS</h1>\n<div class=\"content-section-inner\" id=\"...",
"when_using": null
}
- New Added labels endpoints for getting a specific label, the rendered contents, or historic documents in the United States region.
- New Added endpoints for getting a specific label for a given product or package in the United States region.
- New Added endpoints for getting a list of current labels for a given drug or product concept in the United States region.
March 11, 2021
Get a specific US product
curl -L 'https://api.drugbank.com/v1/products/50090-5181?region=us'
-H 'Authorization: myapikey'
- New Added support for new regions:
- Austria
- Colombia
- Indonesia
- Italy
- Malaysia
- Thailand
- Turkey
- New Added parameter based region filtering for all endpoints. See Selecting your region.
- New Added support for product queries for new region products.
- New Added support for drug-drug interaction queries for new region products.
February 5, 2021
Allergy details
[
{
"source_name": "Penicillins",
"source_type": "categorical",
"summary": "Hypersensitivity to Amoxicillin (as a Penicillin) can present as: Cutaneous Manifestations of Drug Allergy and Erythema multiforme",
"info": "Penicillin allergy is the most common drug allergy, with incidence ranging between five and 25% by geographical region and population. Data is well-described across case reports, retrospective studies, and extensive review of literature since the first reported case in 1945. It is estimated that more than 90% of patients labelled as having a penicillin allergy could safely receive one or more members of this class; penicillin allergy is not durable, and patients lose sensitivity over time. In the case of moderate to high risk patients, allergy testing and/or desensitization prior to treatment is recommended.[A216991, A217016, A214379] In a large prospective case-control study in Europe, penicillins were frequently associated with a risk of erythema multiforme.[A204278]",
"evidence_type": [
"case_reports",
"review"
],
"hypersensitivity_types": [
"Type IV",
"Unclassified"
],
"presentations": [
"Cutaneous Manifestations of Drug Allergy",
"Erythema multiforme"
]
}
]
Cross-sensitivities
[
{
"summary": "As a Penicillin, Amoxicillin is known to be cross-sensitive with Clavulanic acid",
"description": "An allergy to clavulanic acid may lead to an allergy to other beta-lactams, such as penicillins and cephalosporins, due to similarities in chemical structure. According to prescribing information, clavulanate potassium and amoxicillin (in a single product) should not be administered if a patient is allergic to beta-lactam antibiotics.[L7910] Despite this recommendation, some recent studies indicate that clavulanic acid is unlikely to exhibit cross-reactivity with other beta-lactams because clavulanate lacks a side chain in addition to an oxazolidine ring bound to the beta-lactam ring.[A220863,A220893]",
"incidence": "Theoretical",
"evidence_type": [
"review"
],
"cross_sensitive_drugs": [
{
"name": "Clavulanic acid",
"drugbank_id": "DB00766"
}
]
},
{
"summary": "As a Penicillin, Amoxicillin is known to be cross-sensitive with Carbapenems",
"description": "The risk of cross-reactivity between penicillins and carbapenems is less than 1% in patients with a positive penicillin skin test.[A218896] Prospective studies in adults and children with IgE-mediated allergy to penicillin showed that the rate of cross-reactivity with carbapenems (imipenem-cilastatin and meropenem) was 0.9%, after positive skin test results for the two carbapenems. The rate of cross-reactivity with penicillins leading to delayed reactions to carbapenems was 5.5%, based on patients with cell-mediated hypersensitivity to penicillins and positive skin patch tests to at least one penicillin and imipenem-cilastatin.[A220883]",
"incidence": "0.9-5.5%",
"evidence_type": [
"post_marketing",
"review"
],
"cross_sensitive_drugs": [
{
"name": "Biapenem",
"drugbank_id": "DB13028"
},
{
"name": "Doripenem",
"drugbank_id": "DB06211"
},
{
"name": "Ertapenem",
"drugbank_id": "DB00303"
},
{
"name": "Imipenem",
"drugbank_id": "DB01598"
},
{
"name": "Meropenem",
"drugbank_id": "DB00760"
}
]
},
{
"summary": "As a Penicillin, Amoxicillin is known to be cross-sensitive with Cephalosporins",
"description": "Recent studies suggest that cross-sensitivity between penicillins and cephalosporins arise from the similarity between the R1 side chain of earlier generation cephalosporins and penicillins, rather than the ß-lactam structure that they share.[A220508, A220513, A220518] This indicates that cross-sensitivity in a penicillin-allergic patient is not necessarily a class effect; thus, other cephalosporin drugs with dissimilar side chains may be safely considered (for example, a negative skin test result to penicillin determinants).[A214902] Earlier studies report high cross-sensitivity rates between cephalosporins and penicillins that can be as high as 25%.[A220883, A220898] However, more recent studies argue that the cross-sensitivity risk is much lower, with the rate ranging from 0.17% and 0.7%, and 6% in patients with a positive penicillin skin test.[L16458] It is argued that these high rates from earlier reports reflect contamination of manufactured cephalosporin products, cross-reactivity rates based upon non-consecutive case reports, and the fact that aminopenicillins and aminocephalosporins share a common R1 side chain.[A220898] The degree of cross-sensitivity between cephalosporins and penicillins is higher with earlier generation cephalosporins. The risk of cross-sensitivity between penicillins and later (third and fourth) generation cephalosporin is generally low. These newer agents contain less bulky side chains in their structure, resulting in decreased immunogenicity risk.[A214902] The incidence of cross-sensitivity between penicillins and later (third and fourth) generation cephalosporins may be lower than the cross-sensitivity between penicillins and unrelated antibiotics.[A220513]",
"incidence": "\u003c10%",
"evidence_type": [
"uncontrolled_trial",
"review"
],
"cross_sensitive_drugs": [
{
"name": "Cefacetrile",
"drugbank_id": "DB01414"
},
{
"name": "Cefaclor",
"drugbank_id": "DB00833"
},
{
"name": "Cefadroxil",
"drugbank_id": "DB01140"
},
{
"name": "Cefaloridine",
"drugbank_id": "DB09008"
},
{
"name": "Cefalotin",
"drugbank_id": "DB00456"
},
{
"name": "Cefamandole",
"drugbank_id": "DB01326"
},
{
"name": "Cefamandole nafate",
"drugbank_id": "DB14725"
},
{
"name": "Cefapirin",
"drugbank_id": "DB01139"
},
{
"name": "Cefatrizine",
"drugbank_id": "DB13266"
},
{
"name": "Cefazedone",
"drugbank_id": "DB13778"
},
{
"name": "Cefazolin",
"drugbank_id": "DB01327"
},
{
"name": "Cefbuperazone",
"drugbank_id": "DB13638"
},
{
"name": "Cefcapene",
"drugbank_id": "DB13461"
},
{
"name": "Cefetamet",
"drugbank_id": "DB13504"
},
{
"name": "Cefiderocol",
"drugbank_id": "DB14879"
},
{
"name": "Cefmetazole",
"drugbank_id": "DB00274"
},
{
"name": "Cefminox",
"drugbank_id": "DB09062"
},
{
"name": "Cefodizime",
"drugbank_id": "DB13470"
},
{
"name": "Cefonicid",
"drugbank_id": "DB01328"
},
{
"name": "Ceforanide",
"drugbank_id": "DB00923"
},
{
"name": "Cefotetan",
"drugbank_id": "DB01330"
},
{
"name": "Cefotiam",
"drugbank_id": "DB00229"
},
{
"name": "Cefoxitin",
"drugbank_id": "DB01331"
},
{
"name": "Cefpodoxime",
"drugbank_id": "DB01416"
},
{
"name": "Cefprozil",
"drugbank_id": "DB01150"
},
{
"name": "Cefradine",
"drugbank_id": "DB01333"
},
{
"name": "Cefroxadine",
"drugbank_id": "DB11367"
},
{
"name": "Cefsulodin",
"drugbank_id": "DB13499"
},
{
"name": "Ceftaroline fosamil",
"drugbank_id": "DB06590"
},
{
"name": "Ceftezole",
"drugbank_id": "DB13821"
},
{
"name": "Ceftibuten",
"drugbank_id": "DB01415"
},
{
"name": "Ceftobiprole",
"drugbank_id": "DB04918"
},
{
"name": "Ceftolozane",
"drugbank_id": "DB09050"
},
{
"name": "Cefuroxime",
"drugbank_id": "DB01112"
},
{
"name": "Cephalexin",
"drugbank_id": "DB00567"
},
{
"name": "Cephaloglycin",
"drugbank_id": "DB00689"
},
{
"name": "Flomoxef",
"drugbank_id": "DB11935"
},
{
"name": "Latamoxef",
"drugbank_id": "DB04570"
},
{
"name": "Loracarbef",
"drugbank_id": "DB00447"
}
]
},
{
"summary": "As a Beta Lactam R1A, Amoxicillin is known to be cross-sensitive with Beta Lactam R1A",
"description": "Theoretical evidence suggests that beta-lactams sharing the same R1 side chains are likely to result in hypersensitivity reactions caused by cross-reactivity. The R1 side chain is the primary driver of beta-lactam allergy.[A214343] In one study, 12-38% of patients proven to be selectively allergic to amoxicillin, and were tolerant to penicillin, also reacted to cefadroxil.[A214337]",
"incidence": "Theoretical",
"evidence_type": [
"varying_reports",
"review"
],
"cross_sensitive_drugs": [
{
"name": "Cefadroxil",
"drugbank_id": "DB01140"
},
{
"name": "Cefatrizine",
"drugbank_id": "DB13266"
},
{
"name": "Cefprozil",
"drugbank_id": "DB01150"
}
]
}
]
- New Added endpoints for getting allergy details for a given drug, product, or product concept.
- New Added endpoints for getting cross-sensitivities for a given drug, product, or product concept.
November 20, 2020
New fields on product results
{
"allergenic": false,
"cosmetic": true,
"vaccine": false
}
- New Added allergenic, cosmetic, and vaccine booleans to products and drug products endpoints.
November 16, 2020
An example response for
product_concept_id=DBPC0037190,DBPC0005793:
{
"duplicate_ingredients": [
{
"drug": {
"name": "Acetylsalicylic acid",
"drugbank_id": "DB00945"
},
"product_concepts": [
{
"name": "Acetylsalicylic acid / Citric acid / Sodium bicarbonate Tablet, effervescent",
"display_name": null,
"drugbank_pcid": "DBPC0037190",
"brand": null,
"level": 2,
"route": null,
"form": "Tablet, effervescent",
"strengths": null,
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1977-12-31",
"regions": {
"us": true,
"canada": true,
"eu": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Sodium bicarbonate",
"drug": {
"name": "Sodium bicarbonate",
"drugbank_id": "DB01390"
}
},
{
"name": "Citric acid",
"drug": {
"name": "Citric acid",
"drugbank_id": "DB04272"
}
},
{
"name": "Acetylsalicylic acid",
"drug": {
"name": "Acetylsalicylic acid",
"drugbank_id": "DB00945"
}
}
]
},
{
"name": "Acetylsalicylic acid",
"display_name": null,
"drugbank_pcid": "DBPC0005793",
"brand": null,
"level": 1,
"route": null,
"form": null,
"strengths": null,
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1950-12-31",
"regions": {
"us": true,
"canada": true,
"eu": false
},
"rxnorm_concepts": [
{
"name": "acetyl salicylate",
"RXCUI": "91101"
},
{
"name": "aspirin",
"RXCUI": "1191"
}
],
"ingredients": [
{
"name": "Acetylsalicylic acid",
"drug": {
"name": "Acetylsalicylic acid",
"drugbank_id": "DB00945"
}
}
]
}
]
}
],
"duplicate_therapies": [
{
"condition": {
"title": "Headache",
"drugbank_id": "DBCOND0017979"
},
"kind": "management_of",
"duplicates": [
{
"product_concept": {
"name": "Acetylsalicylic acid",
"display_name": null,
"drugbank_pcid": "DBPC0005793",
"brand": null,
"level": 1,
"route": null,
"form": null,
"strengths": null,
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1950-12-31",
"regions": {
"us": true,
"canada": true,
"eu": false
},
"rxnorm_concepts": [
{
"name": "acetyl salicylate",
"RXCUI": "91101"
},
{
"name": "aspirin",
"RXCUI": "1191"
}
],
"ingredients": [
{
"name": "Acetylsalicylic acid",
"drug": {
"name": "Acetylsalicylic acid",
"drugbank_id": "DB00945"
}
}
]
},
"indication": {
"kind": "used_in_combination_to_manage",
"off_label": true,
"otc_use": false,
"combination_type": "regimen",
"drug": {
"name": "Acetylsalicylic acid",
"drugbank_id": "DB00945"
},
"condition": {
"name": "Headache",
"drugbank_id": "DBCOND0017979",
"meddra_id": "hlt/10019233",
"snomed_id": "c/206946005",
"icd10_id": "c/R51"
},
"combination_drugs": [
{
"name": "Dextropropoxyphene",
"drugbank_id": "DB00647"
}
]
}
},
{
"product_concept": {
"name": "Acetylsalicylic acid / Citric acid / Sodium bicarbonate Tablet, effervescent",
"drugbank_pcid": "DBPC0037190",
"level": 2,
"form": "Tablet, effervescent",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1977-12-31",
"regions": {
"us": true,
"canada": true,
"eu": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Sodium bicarbonate",
"drug": {
"name": "Sodium bicarbonate",
"drugbank_id": "DB01390"
}
},
{
"name": "Citric acid",
"drug": {
"name": "Citric acid",
"drugbank_id": "DB04272"
}
},
{
"name": "Acetylsalicylic acid",
"drug": {
"name": "Acetylsalicylic acid",
"drugbank_id": "DB00945"
}
}
]
},
"indication": {
"kind": "used_in_combination_to_manage",
"off_label": false,
"otc_use": true,
"combination_type": "product",
"drug": {
"name": "Citric acid",
"drugbank_id": "DB04272"
},
"regions": "US",
"condition": {
"name": "Headache",
"drugbank_id": "DBCOND0017979",
"meddra_id": "hlt/10019233",
"snomed_id": "c/206946005",
"icd10_id": "c/R51"
},
"combination_drugs": [
{
"name": "Acetylsalicylic acid",
"drugbank_id": "DB00945"
},
{
"name": "Sodium bicarbonate",
"drugbank_id": "DB01390"
}
]
}
}
]
}
]
}
- New Added
/product_concepts/duplicate_therapiesendpoint to check whether given product concept ids contain duplicate ingredients or address similar conditions.
November 2, 2020
- New Added generic product filter to the drug products endpoint.
- New Added endpoint for finding generic products, given a starting product.
- New Added endpoints for finding product concepts with similar indications to a given product concept or a product.
September 23, 2020
- New Added
ema_ma_numbersto Drug Names search results.
September 17, 2020
- New Added Product Concept indication filtering option
When querying indications for a product or product concept, you can now set an adjunct_use flag to true or false. If set to false, only directly related indications (those involving the main drug) of the product will be returned. If set to true, only indirect indications (those that don’t involve the main drug) will be returned. If left unset, both direct and indirect indications will be returned, the same behaviour as prior to this update.
August 24, 2020
- New Product Concept indication queries will now return more complete results.
Indication query results for certain product concepts have been improved. In particular, product concepts like Acyclovir 5% (DBPC0119485) would previously omit certain indications, such as an indication for Topical Acyclovir, from their indication query results. All product concept indication queries will now return all relevant results.
August 21, 2020
An example response including the ATC combination code:
[
{
"code": "N07BC02",
"title": "methadone",
"combination": false
},
{
"code": "N02AC52",
"title": "methadone, combinations excl. psycholeptics",
"combination": true
}
]
- New Drug to ATC mappings will now indicate if the ATC code is a combination code (i.e. more than one drug), or a single drug code.
August 6, 2020
An example response including the NDC full package description:
{
"package_ndc_code": "11523-4329-01",
"originator_package_ndc_code": "11523-4329-1",
"description": "2 CARTON IN 1 PACKAGE, COMBINATION",
"full_description": "2 CARTON IN 1 PACKAGE, COMBINATION > 3 BLISTER PACK IN 1 CARTON > 10 TABLET, ORALLY DISINTEGRATING IN 1 BLISTER PACK"
}
- New The NDC Package API endpoint now includes the full package description in the same format used by the FDA/NDC. Regardless of what level of package your are accessing, the full description, including the outer packaging, will be present.